Abstract
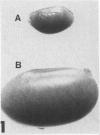
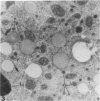
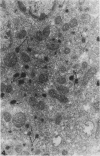
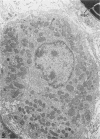
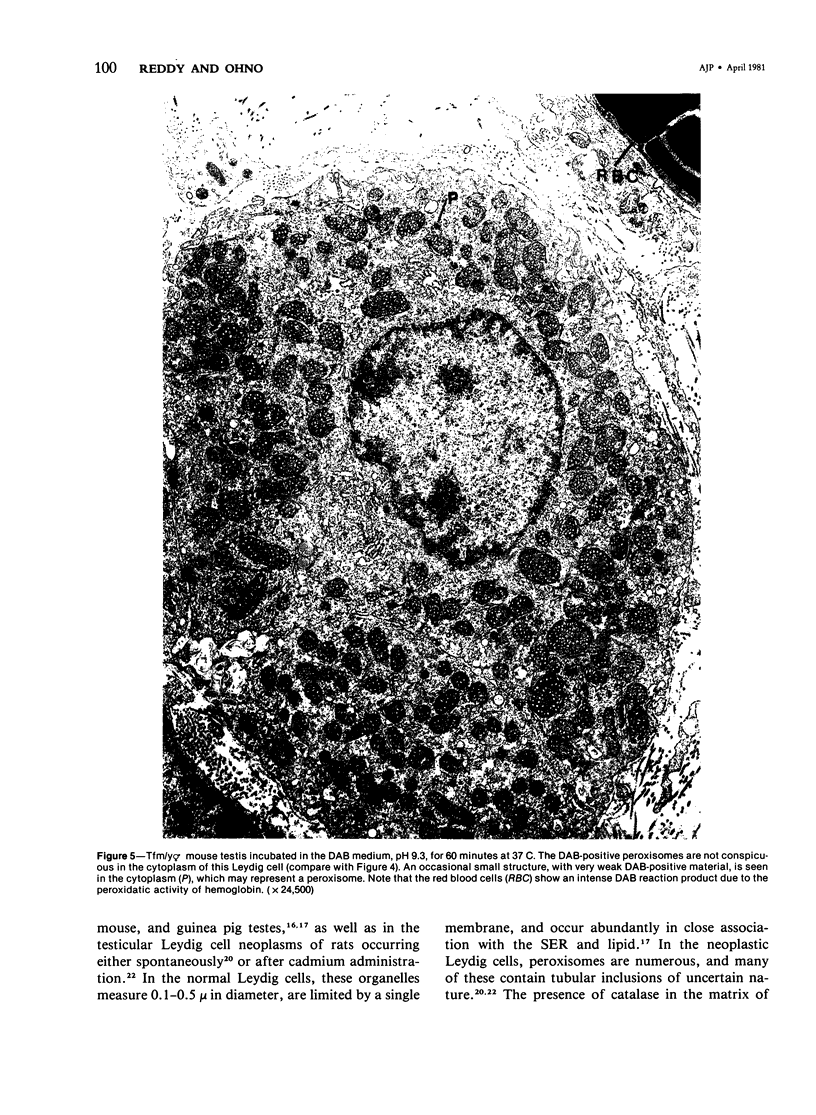
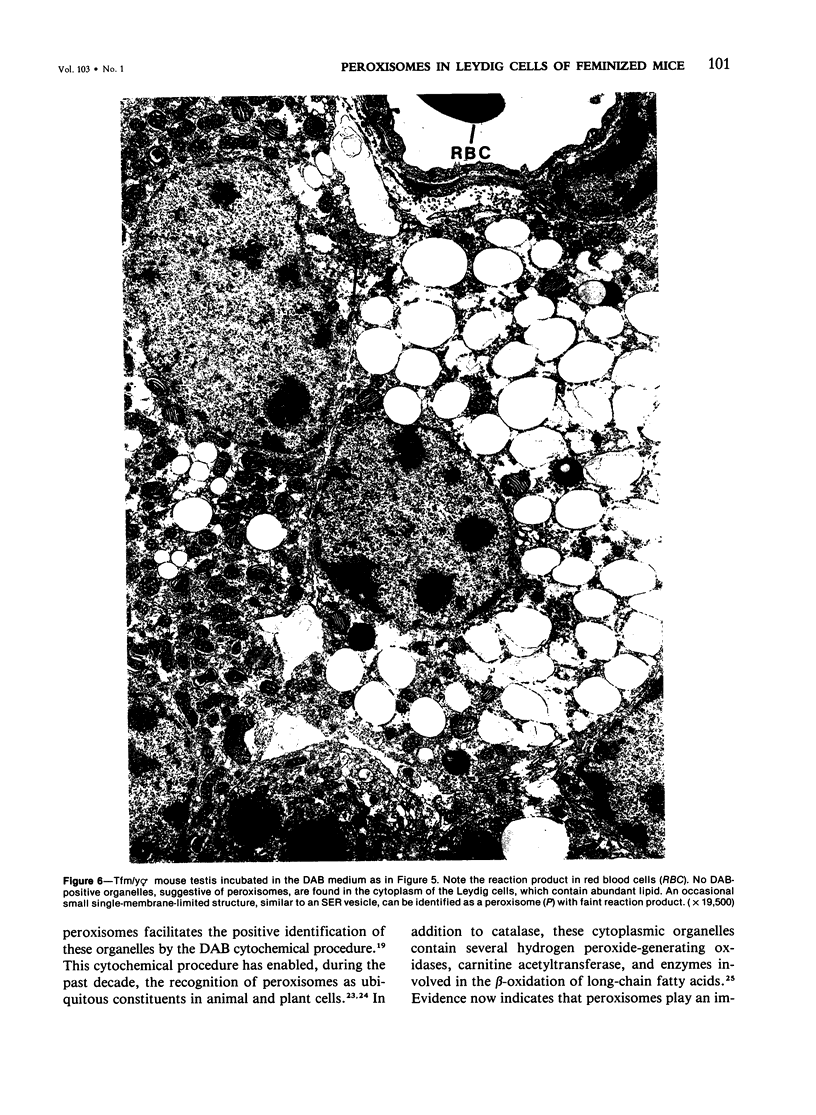
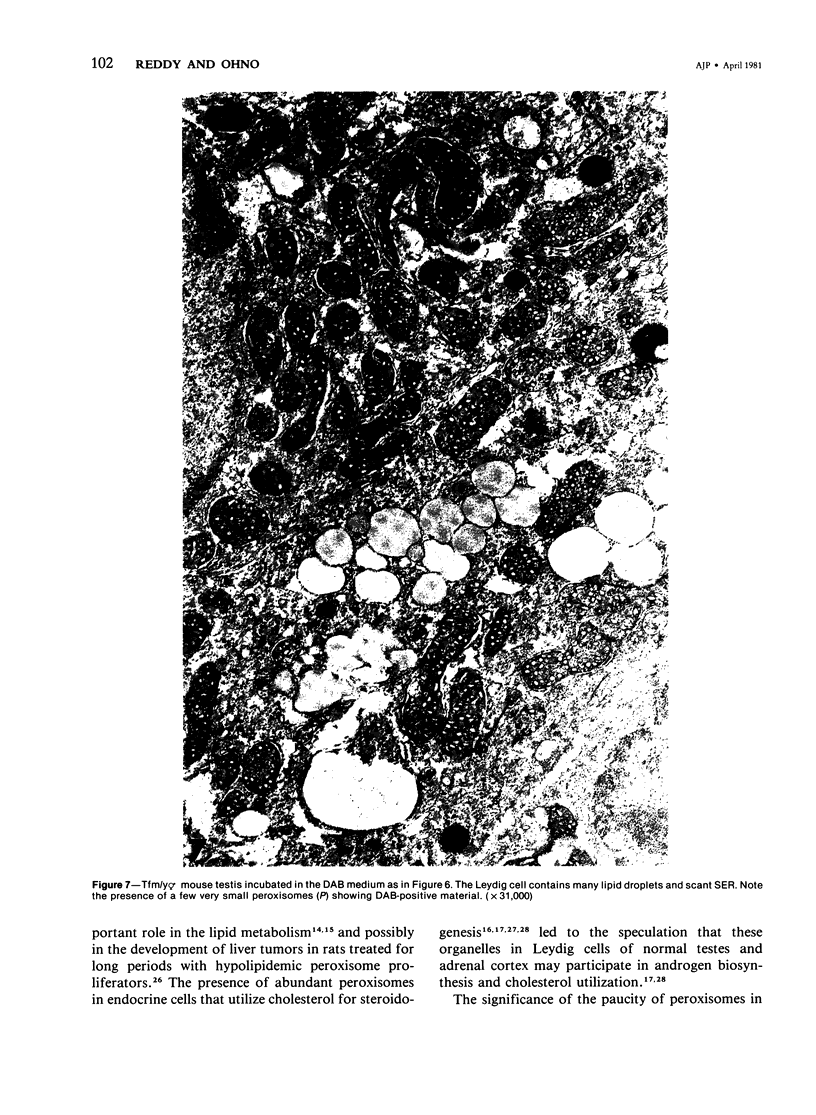
The testes of mice with the X-linked testicular feminization (Tfm/y hermaphrodite) mutation are very small and cryptorchid. The spermatogenesis in the adult Tfm/y hermaphrodite mouse testes is arrested, and the testosterone biosynthesis is significantly reduced due to deficiency of 17-keto-steroid reductase, the enzyme essential for the conversion of androstanedione to testosterone. In this study the distribution of peroxisomes in the Leydig cells of adult Tfm/y hermaphrodite mice was investigated because of the suggestion that peroxisomes may participate in lipid metabolism and/or androgen biosynthesis is steroidogenic cells. Aldehyde-fixed testicular tissue of Tfm/y hermaphrodite mice was processed for the cytochemical localization of peroxisome catalase to facilitate identification of these organelles in the Leydig cells. Testes from Blo/y and CSa strain normal adult mice served as controls. Testicular Leydig cells of normal adult Blo/y and CSa mice contained abundant smooth endoplasmic reticulum (SER) in the form of complex interconnected tubules and double-walled membranous vesicles. Numerous peroxisomes, often in continuity with SER channels or in close association with lipid droplets, were observed in Leydig cells of normal males. In contrast, the peroxisomes in the Leydig cells of adult Tfm/y hermaphrodite mouse testes were either undiscernible or greatly reduced in number and size. SER in these cells was sparse, whereas mitochondria were numerous. In addition, abundant clusters of lipid droplets were encountered in a majority of Leydig cells of Tfm/y hermaphrodite mouse testes. Peroxisome and SER paucity in Leydig cells of Tfm/y hermaphrodite mice may be a reflection of reduced testosterone production. Whether excessive accumulation of lipid in the Leydig cells of Tfm/y hermaphrodite mouse testes is due to reduced utilization of cholesterol for the biosynthesis of testosterone or to impaired lipid metabolism due to reduction in peroxisome population in these cells remains to be ascertained.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardin C. W., Bullock L. P., Sherins R. J., Mowszowicz I., Blackburn W. R. Androgen metabolism and mechanism of action in male pseudohermaphroditism: a study of testicular feminization. Recent Prog Horm Res. 1973;29:65–109. doi: 10.1016/b978-0-12-571129-6.50006-3. [DOI] [PubMed] [Google Scholar]
- Bardin C. W., Bullock L., Schneider G., Allison J. E., Stanley A. J. Pseudohermaphrodite rat: end organ insensitivity to testosterone. Science. 1970 Feb 20;167(3921):1136–1137. doi: 10.1126/science.167.3921.1136. [DOI] [PubMed] [Google Scholar]
- Beard M. E. Identification of peroxisomes in the rat adrenal cortex. J Histochem Cytochem. 1972 Mar;20(3):173–179. doi: 10.1177/20.3.173. [DOI] [PubMed] [Google Scholar]
- Black V. H., Bogart B. I. Peroxisomes in inner adrenocortical cells of fetal and adult guinea pigs. J Cell Biol. 1973 May;57(2):345–358. doi: 10.1083/jcb.57.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn W. R., Chung K. W., Bullock L., Bardin C. W. Testicular feminization in the mouse: studies of Leydig cell structure and function. Biol Reprod. 1973 Aug;9(1):9–23. doi: 10.1093/biolreprod/9.1.9. [DOI] [PubMed] [Google Scholar]
- Christensen A. K., Fawcett D. W. The fine structure of testicular interstitial cells in mice. Am J Anat. 1966 Mar;118(2):551–571. doi: 10.1002/aja.1001180214. [DOI] [PubMed] [Google Scholar]
- Chung K. W., Allison J. E., Stanley A. J. Structural and functional factors related to testicular neoplasia in feminized rats. J Natl Cancer Inst. 1980 Jul;65(1):161–168. [PubMed] [Google Scholar]
- Chung K. W., Allison J. E. Ultrastructural and biochemical characteristics of Leydig cells from newborn androgen-insensitive rats. Cell Tissue Res. 1979 Feb 15;196(2):213–219. doi: 10.1007/BF00240097. [DOI] [PubMed] [Google Scholar]
- Chung K. W., Hamilton J. B. Further observations on the fine structure of Leydig cells in the testes of male pseudohermaphrodite rats. J Ultrastruct Res. 1976 Jan;54(1):68–75. doi: 10.1016/s0022-5320(76)80009-3. [DOI] [PubMed] [Google Scholar]
- Chung K. W., Hamilton J. B. Testicular lipids in mice with testicular feminization. Cell Tissue Res. 1975 Jun 27;160(1):69–80. doi: 10.1007/BF00219842. [DOI] [PubMed] [Google Scholar]
- Chung K. W. Spontaneous testicular neoplasm in mice with testicular feminization. Cell Tissue Res. 1980;208(1):47–56. doi: 10.1007/BF00234172. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Wilson J. D. Studies on the pathogenesis of the pseudohermaphroditism in the mouse with testicular feminization. J Clin Invest. 1972 Jul;51(7):1647–1658. doi: 10.1172/JCI106966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruban Z., Vigil E. L., Slesers A., Hopkins E. Microbodies: constituent organelles of animal cells. Lab Invest. 1972 Aug;27(2):184–191. [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F., Hawkes S. G. X-linked gene for testicular feminization in the mouse. Nature. 1970 Sep 19;227(5264):1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- MORRIS J. M. The syndrome of testicular feminization in male pseudohermaphrodites. Am J Obstet Gynecol. 1953 Jun;65(6):1192–1211. doi: 10.1016/0002-9378(53)90359-7. [DOI] [PubMed] [Google Scholar]
- Masters C., Holmes R. Peroxisomes: new aspects of cell physiology and biochemistry. Physiol Rev. 1977 Oct;57(4):816–882. doi: 10.1152/physrev.1977.57.4.816. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. V. Are microperoxisomes ubiquitous in mammalian cells? J Histochem Cytochem. 1973 Aug;21(8):737–755. doi: 10.1177/21.8.737. [DOI] [PubMed] [Google Scholar]
- Ono S. Animal model of human disease. Testicular feminization. Am J Pathol. 1974 Sep;76(3):589–592. [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Azarnoff D. L., Hignite C. E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980 Jan 24;283(5745):397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Krishnakantha T. P. Hepatic peroxisome proliferation: induction by two novel compounds structurally unrelated to clofibrate. Science. 1975 Nov 21;190(4216):787–789. doi: 10.1126/science.1198095. [DOI] [PubMed] [Google Scholar]
- Reddy J. K. Possible properties of microbodies (peroxisomes). Microbody proliferation and hypolipidemic drugs. J Histochem Cytochem. 1973 Nov;21(11):967–971. doi: 10.1177/21.11.967. [DOI] [PubMed] [Google Scholar]
- Reddy J., Svoboda D., Azarnoff D., Dawar R. Cadmium-induced Leydig cell tumors of rat testis: morphologic and cytochemical study. J Natl Cancer Inst. 1973 Sep;51(3):891–903. doi: 10.1093/jnci/51.3.891. [DOI] [PubMed] [Google Scholar]
- Reddy J., Svoboda D. Microbodies (peroxisomes) identification in interstitial cells of the testis. J Histochem Cytochem. 1972 Feb;20(2):140–142. doi: 10.1177/20.2.140. [DOI] [PubMed] [Google Scholar]
- Reddy J., Svoboda D. Microbodies (peroxisomes) in the interstitial cells of rodent testes. Lab Invest. 1972 Jun;26(6):657–665. [PubMed] [Google Scholar]
- Reddy J., Svoboda D. Microbodies in Leydig cell tumors of rat testis. J Histochem Cytochem. 1972 Oct;20(10):793–803. doi: 10.1177/20.10.793. [DOI] [PubMed] [Google Scholar]
- Reddy J., Svoboda D. Microbodies in experimentally altered cells. 8. Continuities between microbodies and their possible biologic significance. Lab Invest. 1971 Jan;24(1):74–81. [PubMed] [Google Scholar]