Abstract
The formation of spironolactone (S) bodies, eosinophilic laminated cytoplasmic inclusions, is induced in the aldosterone-producing cells of the human adrenal cortex after the administration of spironolactone. The aim of this study was to define the enzyme histochemical characteristics of S bodies, S-body-containing cells, and the apparently hyperplastic zona glomerulosa (zG) of adrenal tissues attached to aldosteronomas. S bodies were found in 14 of 19 aldosteronomas, in 10 of 19 adrenal tissues attached to aldosteronomas, and in the adrenal tissues in a patient with aldosteronism due to bilateral diffuse zG hyperplasia. The S bodies themselves exhibited most intense 3 beta-hydroxysteroid dehydrogenase (3 beta HSD) activity but did not exhibit glucose-6-phosphate dehydrogenase (G6PD), NADP-dependent isocitrate dehydrogenase (NADP-ICDH), or succinate dehydrogenase (SDH) activity, confirming histochemically the origin of S bodies in the smooth endoplasmic reticulum. In two adenomas, S bodies were found to be surrounded by reaction products of acid hydrolase but were not found in the other adenomas and the remaining adrenal tissues. S-body-containing cells, irrespective of being neoplastic or not, showed enhanced 3 beta HSD, G6PD, and NADP-ICDH activity and weak SDH activity (Type I pattern of enzyme activity). Though zG was hyperplastic in most of the adrenal tissues attached to the adenomas, zG cells that did not contain S bodies showed the opposite pattern (Type II pattern) of enzyme activity (ie, weak 3 beta HSD, G6PD, and NADP-ICDH activity and intense SDH activity), in contrast to those in the adrenal tissues in a patient with aldosteronism due to bilateral diffuse zG hyperplasia (which showed the Type I pattern). The results are consistent with the view that hyperplastic zG cells, except S-body-containing cells, in the case of aldosteronoma are not hyperfunctioning. The latter cells may have enhanced but possibly abortive steroidogenic activity.
Full text
PDF

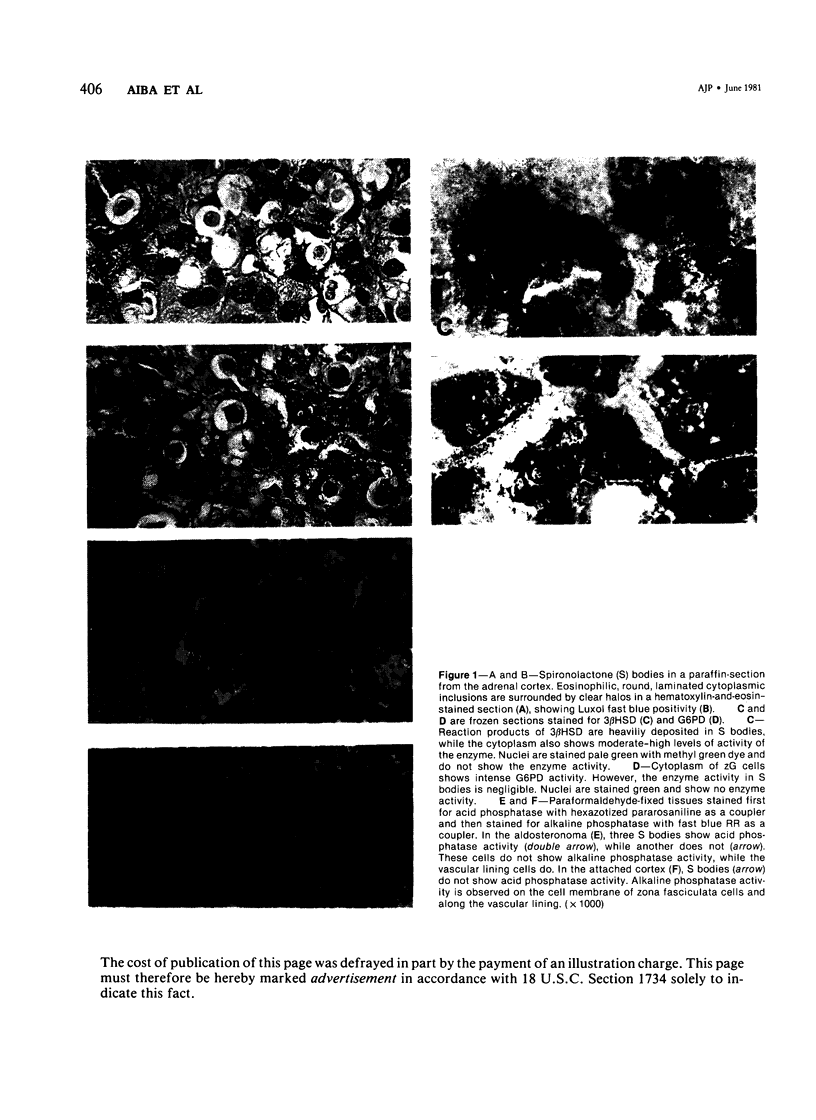




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba M., Kameya T., Suzuki H., Nakamura H., Mizuno Y., Kanno T. Enzyme histochemical and electron microscopic study of a virilizing adrenocortical adenoma. Acta Pathol Jpn. 1978 Jul;28(4):615–626. doi: 10.1111/j.1440-1827.1978.tb00900.x. [DOI] [PubMed] [Google Scholar]
- BURSTONE M. S., KEYES P. H. Studies on calcification. I. The effect of inhibition of enzyme activity on developing bone and dentin. Am J Pathol. 1957 Nov-Dec;33(6):1229–1235. [PMC free article] [PubMed] [Google Scholar]
- Cain D. R., Van de Velde R. L., Shapiro S. J. Spironolactone inclusions in an aldosteronoma. Am J Clin Pathol. 1974 Mar;61(3):412–416. doi: 10.1093/ajcp/61.3.412. [DOI] [PubMed] [Google Scholar]
- Cheng S. C., Suzuki K., Sadee W., Harding B. W. Effects of spironolactone, canrenone and canrenoate-K on cytochrome P450, and 11beta- and 18-hydroxylation in bovine and human adrenal cortical mitochondria. Endocrinology. 1976 Oct;99(4):1097–1106. doi: 10.1210/endo-99-4-1097. [DOI] [PubMed] [Google Scholar]
- Conn J. W., Hinerman D. L. Spironolactone-induced inhibition of aldosterone biosynthesis in primary aldosteronism: morphological and functional studies. Metabolism. 1977 Dec;26(12):1293–1307. doi: 10.1016/0026-0495(77)90026-9. [DOI] [PubMed] [Google Scholar]
- Davis D. A., Medline N. M. Spironolactone (aldactone) bodies: concentric lamellar formations in the adrenal cortices of patients treated with spironolactone. Am J Clin Pathol. 1970 Jul;54(1):22–32. doi: 10.1093/ajcp/54.1.22. [DOI] [PubMed] [Google Scholar]
- Erbler H. C. The effect of saluretics and spironolactone on aldosterone production and electrolyte excretion in man. Naunyn Schmiedebergs Arch Pharmacol. 1974;286(2):145–156. doi: 10.1007/BF00501608. [DOI] [PubMed] [Google Scholar]
- HAYASHI M. HISTOCHEMICAL DEMONSTRATION OF N-ACETYL-BETA-GLUCOSAMINIDASE EMPLOYING NAPHTHOL AS-BI N-ACETYL-BETA -GLUCOSAMINIDE AS SUBSTRATE. J Histochem Cytochem. 1965 May-Jun;13:355–360. doi: 10.1177/13.5.355. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., NAKAJIMA Y., FISHMAN W. H. THE CYTOLOGIC DEMONSTRATION OF BETA-GLUCURONIDASE EMPLOYING NAPHTHOL AS-BI GLUCURONIDE AND HEXAZONIUM PARAROSANILIN; A PRELIMINARY REPORT. J Histochem Cytochem. 1964 Apr;12:293–297. doi: 10.1177/12.4.293. [DOI] [PubMed] [Google Scholar]
- Headon D. R., Hsiao J., Ungar F. The intracellular localization of adrenal 3beta-hydroxysteroid dehydrogenase/delta5-isomerase by density gradient perturbation. Biochem Biophys Res Commun. 1978 Jun 14;82(3):1006–1012. doi: 10.1016/0006-291x(78)90883-5. [DOI] [PubMed] [Google Scholar]
- JANIGAN D. T. Cytoplasmic bodies in the adrenal cortex of patients treated with spirolactone. Lancet. 1963 Apr 20;1(7286):850–852. doi: 10.1016/s0140-6736(63)91624-6. [DOI] [PubMed] [Google Scholar]
- Jenis E. H., Hertzog R. W. Effect of spironolactone on the zona. Light and electron microscopy. Arch Pathol. 1969 Nov;88(5):530–539. [PubMed] [Google Scholar]
- Kovacs K., Horvath E., Singer W. Fine structure and morphogenesis of spironolactone bodies in the zona glomerulosa of the human adrenal cortex. J Clin Pathol. 1973 Dec;26(12):949–957. doi: 10.1136/jcp.26.12.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M. Histochemical evaluation of NADP-dependent glucose-6-phosphate and isocitrate dehydrogenases in steroid producing organs and tumors. Arch Histol Jpn. 1967 Feb;28(1):45–57. doi: 10.1679/aohc1950.28.45. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Okano K., Matsumoto K., Koizumi T., Mizushima T., Mori M. Histochemical comparison of oxidative enzymes in adrenal glands of mammals. Histochemie. 1965 Mar 5;4(6):494–501. doi: 10.1007/BF00281902. [DOI] [PubMed] [Google Scholar]
- Shrago S. S., Waisman J., Cooper P. H. Spironolactone bodies in an adrenal adenoma. Arch Pathol. 1975 Aug;99(8):416–420. [PubMed] [Google Scholar]
- Sundsfjord J. A., Marton P., Jorgensen H., Aakvaag A. Reduced aldosterone secretion during spironolactone treatment in primary aldosteronism: report of a case. J Clin Endocrinol Metab. 1974 Oct;39(4):734–739. doi: 10.1210/jcem-39-4-734. [DOI] [PubMed] [Google Scholar]
- WATTENBERG L. W. Microscopic histochemical demonstration of steroid-3 beta-ol dehydrogenase in tissue sections. J Histochem Cytochem. 1958 Jul;6(4):225–232. doi: 10.1177/6.4.225. [DOI] [PubMed] [Google Scholar]




