Abstract
In mammals, including humans, female fetuses that are exposed to testosterone from adjacent male fetuses in utero can have masculinized anatomy and behavior. However, the reproductive consequences of such prebirth sex-ratio effects for offspring and their implications for maternal fitness remain unexplored. Here we investigate the effects of being gestated with a male co-twin for daughter lifetime reproductive success, and the fitness consequences for mothers of producing mixed-sex twins in preindustrial (1734–1888) Finns. We show that daughters born with a male co-twin have reduced lifetime reproductive success compared to those born with a female co-twin. This reduction arises because such daughters have decreased probabilities of marrying as well as reduced fecundity. Mothers who produce opposite-sex twins consequently have fewer grandchildren (and hence lower fitness) than mothers who produce same-sex twins. Our results are unlikely to be a consequence of females born with male co-twins receiving less nutrition because such females do not have reduced survival and increases in food availability fail to improve their reproductive success. Nor are our results explained by after-birth social factors (females growing up with similarly aged brothers) because females born with a male co-twin have reduced success even when their co-twin dies shortly after birth and are raised as singletons after birth. Our findings suggest that hormonal interactions between opposite-sex fetuses known to influence female morphology and behavior can also have negative effects on daughter fecundity and, hence, maternal fitness, and bear significant implications for adaptive sex allocation in mammals.
Keywords: early conditions, fetal testosterone, reproductive success, sex allocation, sex ratio
Conditions experienced before birth can have profound effects on the subsequent growth, survival, and reproductive capacity of individuals from a wide range of taxa (1, 2). For example, in humans, the quality and quantity of nutrition received in utero and/or the seasonal timing of birth have been shown to predict postnatal growth rates (3), the onset of chronic diseases in adulthood (4), longevity (5, 6), and reproductive success or fitness (7, 8). Another, but less often considered, aspect of the early environment that can have significant downstream consequences for offspring is the amount of sex hormones (testosterones and estrogens) to which developing young are exposed (9–11).
Testosterone and estrogen are fat-soluble steroids and therefore can be transported both in the bloodstream as well as by diffusion. Consequently, it is now well established that, in viviparous animals, sex hormones diffuse through fetal membranes and amniotic fluid, leading to fetuses receiving significant concentrations of sex hormones from their developing neighbors (9, 12). Such acquisition has been shown to have substantial consequences for individual morphology, physiology, and behavior across a wide range of wild, domesticated, and laboratory mammals, as well as in humans (9–11).
In rodents, female fetuses positioned between two males have higher levels of testosterone than those from the same litter positioned between females (9). Furthermore, such females commonly have greater (i.e., more male-like) anogenital distances and often show more aggression, delayed maturation, longer estrus cycles, reduced sexual attractiveness to males, and shorter reproductive lifespans (9). Given that testosterone is lipid-soluble, there is no basis for assuming that interfetal transfer of testosterone, unequivocally demonstrated in rodents, will not occur in any multiparous mammal. Thus, in humans, sex hormones are also likely to diffuse across fetal membranes and amniotic fluid, leading to the likelihood that human twins also can be influenced hormonally by the presence of a co-twin (13). In accordance, human twin studies have shown that having a male co-twin can be associated with increased female growth in utero (14, 15) and masculinization of sexually dimorphic anatomical traits known to be sensitive to testosterone concentrations during fetal development, including second- to fourth-digit finger ratio (16), auditory system (17), craniofacial growth (18), visual acuity (19), and canine size (20). In addition, such females commonly show more male-like behaviors and attitudes after birth (11).
Therefore, a male cosibling can have significant effects on the subsequent morphology and behavior of females in humans and other mammals. However, it is currently unknown whether hormonal interactions between fetuses can also have consequences for offspring reproductive success and maternal fitness in humans or natural populations of wild animals (10), although evidence in laboratory rodents suggests that this might be the case (21). Such studies are rare in wild animals because of the difficulties of following a whole litter of offspring throughout their life in nature and recording all reproductive events. Nevertheless, understanding the fitness consequences of offspring sex ratios has significant consequences for our understanding of the strength of selection on, and hence the evolution of, adaptive sex allocation in viviparous animals. For example, maternal fitness returns on reproductive investments depend, in part, on the future reproductive value of the offspring produced. Consequently, if litter sex ratios influence the survival and reproductive success of offspring through interactions among siblings in utero, this could influence selection on maternal sex ratios in ways which are seldom considered (10).
The aim of this study is to test the hypothesis that the presence of an opposite- versus same-sex co-twin influences lifetime reproductive success in humans, using demographic data from five discrete populations of preindustrial Finns living in conditions of natural mortality and fertility (without benefit of advanced health care and contraception, 1734–1888) (22, 23). In humans, male fetuses have higher levels of testosterone, but similar levels of estrogen, compared to female fetuses. Consequently, males with an opposite-sex co-twin may be exposed to similar levels of estrogens as males with a same-sex co-twin, but females with an opposite-sex co-twin may be exposed to elevated levels of testosterone compared with females with a same-sex co-twin (24). We therefore predict that the consequences of receiving sex hormones from opposite-sexed neighbors will be greater for females. First, we investigate the effect of having an opposite-sex co-twin for an individual's future lifetime reproductive success, measured as both the probability of rearing at least one offspring in a lifetime and the number of offspring successfully reared to adulthood (age 15 years). Second, we investigate the underlying life-history traits that influence twin lifetime reproductive success (their own survival probability to adulthood, marriage probability, and lifetime fecundity, as well as the survival probability of the twin's own offspring to adulthood at age 15 years). Third, to rule out the possibility that after-birth social factors (rather than prenatal exposure to hormones from the opposite-sex co-twin) account for any differences found, we compare the success of males and females born with an opposite-versus same-sex co-twin but raised as singletons after birth because of their co-twin dying within 3 months after birth. Finally, we investigate the effects of producing twins of differing sex ratios for maternal fitness (i.e., their number of grandchildren born).
We use humans from the preindustrial era because survival and reproductive data on modern Western societies is compromised by advanced health care and contraception; although fitness effects have not been considered, studies from modern Western societies not surprisingly show mixed evidence for the hypothesis that female developmental rates or fertility are adversely affected by having a male co-twin (25–28). It is therefore likely that long-term effects of fetal hormonal experiences on later life-history traits and fitness are most effectively investigated in populations living in premodern conditions in which modern contraceptive and medical advances are lacking. Our study includes an extensive dataset with complete reproductive histories of all twins born, their parents, and their resulting offspring from five geographically distinct parishes in rural Finland born between 1734 and 1888 (n = 754 twin offspring). Among these, dizygotic twin births were six times more common than monozygotic twins (see Materials and Methods). All analyses control for necessary potential influences of geographic and temporal variation in fertility and mortality, maternal age and parity, number of competing siblings, and food availability, which was measured as family wealth (social class) and whether birth place was in famine-prone inland parishes or food-predictable island parishes (see Materials and Methods).
Results
Effects of Co-Twin Sex on Reproductive Success.
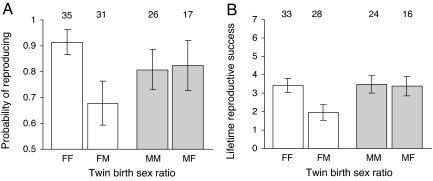
Our results show that females who had a male co-twin have reduced fitness compared to those who had a female co-twin, but the success of males is unaffected by the sex of their co-twin. First, we found that twin females who survived to adulthood have a 25% reduced probability of producing any offspring in their lifetimes if they had a male co-twin rather than a female co-twin (logistic regression: χ2 = 6.68, P = 0.0098; Fig. 1A). Second, of those twin females who married, those who had a male co-twin rear significantly fewer offspring to adulthood in their lifetimes than those who had a female co-twin [general linear model (GLM): F1,59 = 7.22, P = 0.0093; Fig. 1B]. By contrast, males who survived to adulthood have a similar probability of producing any offspring in their lifetimes (logistic regression: χ2 = 0.02, P = 0.90; Fig. 1A) and produce similar numbers of surviving offspring to adulthood (GLM; F1,37 = 0.02, P = 0.89; Fig. 1B), irrespective of whether they had a female or male co-twin.
Fig. 1.
Effect of having a same-sex versus opposite-sex co-twin for female (white bars) and male (gray bars) fitness in premodern Finland. (A) Probability of reproducing in one's lifetime. (B) Lifetime reproductive success measured as the total number of offspring raised to adulthood (age 15 years). FF, females from female–female twin births; FM, females from female–male twin births; MM, males from male–male twin births; MF, males from male–female twin births. Bars indicate predicted means (±1 SE); numbers above the bars indicate sample sizes. Note that the true difference in final fitness between FM and FF females will be even larger than shown in B, given that unmarried women (who were more likely to be FM) with zero numbers of children were not included in the analyses of the effects of having a same- versus opposite-sex co-twin on the numbers of children born and raised.
Effects of Co-Twin Sex on Underlying Life-History Traits.
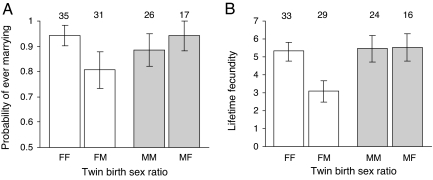
The reduction in female reproductive success above is a consequence of having had a male co-twin on both their probability of marrying and their fecundity, but not on either their survival or the survival of their offspring. First, females who had a male co-twin have a reduced probability of ever marrying in their lifetimes compared to those who had a female co-twin (logistic regression: χ2 = 4.15, P = 0.042; Fig. 2A). This is significant because, in our study population, females who remained unmarried seldom produced any children in their lifetimes (although offspring of unwed mothers were regularly recorded in the church books); only a single twin daughter in our sample who remained unmarried throughout her life reproduced. Second, among those females who did marry, those who had a male co-twin delivered significantly (at least two) fewer offspring in their lifetimes compared to those who had a female co-twin (GLM: F1,59 = 8.06, P = 0.0062; Fig. 2B). However, females with male versus female co-twins have similar survival probability to adulthood (age 15 years) (Cox proportional hazards regression: χ2 = 0.1, P = 0.75, n = 364) and similar lifespans after age 15 years (GLM; F1,71 = 0.38, P = 0.54). These survival results indicate that the differences observed in the probability of marrying and in the numbers of children delivered (see above) are not influenced by differential mortality between females who had same- versus opposite-sex co-twins. Similarly, the survival probability (to age 15 years) of the offspring delivered by those females who had a male versus female co-twin does not differ (χ2 = 0.19, P = 0.66), suggesting that differences in the numbers of offspring reared to adulthood above reflect differences in female fertility rather than offspring viability (or living conditions).
Fig. 2.
Effects of having a same- versus opposite-sex co-twin for female (white bars) and male (gray bars) underlying life-history traits responsible for differences in fitness in Fig. 1. (A) Probability of ever marrying in a lifetime. (B) Lifetime fecundity measured as the total number of offspring delivered. Analyses for the effects on marriage probability only include individuals who survived to the age in which 90% of individuals in a population were married, if they were ever to marry in their lifetime (30 years). FF, females from female–female twin births; FM, females from female–male twin births; MM, males from male–male twin births; MF, males from male–female twin births. Bars indicate predicted means (±1 SE); numbers above the bars indicate sample sizes.
In contrast to females, none of the life-history traits considered differ between males who had a male versus female co-twin (all P > 0.5). In particular, male twins are as likely to marry (χ2 = 0.41, P = 0.52; Fig. 2A) and the wives of married men produce similar numbers of offspring (F1,41 = 0.02, P = 0.90; Fig. 2B), irrespective of whether the men had a male or female co-twin.
Prenatal Versus Postnatal Effects of Co-Twins.
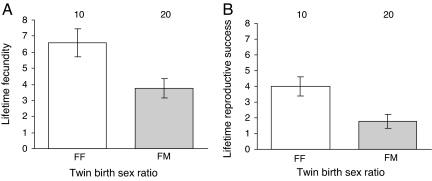
To investigate the possibility that the significant results above are a consequence of postnatal social or nutritional effects, we compared the success of male and female twins who were born with an opposite- versus same-sex co-twin, but who were subsequently reared as singletons after birth as a consequence of their respective co-twin dying within 3 months after birth. Females born with a male co-twin but raised as singletons after birth still have impaired lifetime fecundity (F1,28 = 7.15, P = 0.012; Fig. 3A) and lifetime reproductive success (F1,28 = 7.98, P = 0.008; Fig. 3B) compared to females born with a female co-twin and raised as singletons. Again, there are no differences in the lifetime number of children born or raised between males born with a male or female co-twin and who were raised as singletons (fecundity, F1,10 = 1.92, P = 0.20; lifetime reproductive success, F1,11 = 1.23, P = 0.29).
Fig. 3.
Effect of the death of a co-twin within 3 months after birth on the fitness of the survivor. (A) Lifetime fecundity. (B) Lifetime reproductive success of twin females raised as singletons since birth, but born with a same-sex (FF) or opposite-sex (FM) co-twin. Bars indicate predicted means (±1 SE); numbers above bars indicate sample sizes.
Effects of Twin Sex Ratio on Maternal Fitness.
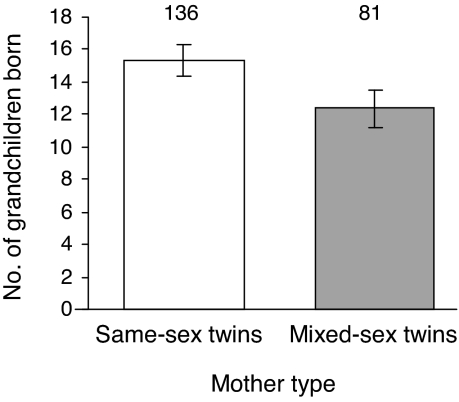
The negative effects of the presence of an opposite-sexed co-twin on the lifetime reproductive success of female offspring have consequences for maternal fitness. Mothers who produced opposite-sex twins have significantly fewer grandchildren than mothers who produced same-sex twins (F1,209 = 5.10, P = 0.025; Fig. 4). This effect is consistent across geographic areas, cohorts, and social classes and is also significant after controlling for differing numbers of offspring raised to adulthood (see Discussion).
Fig. 4.
Effect of producing same- versus mixed-sex twins on the mother's fitness, measured as the number of grandchildren produced by her offspring (controlling for differing numbers of offspring raised to adulthood). Bars indicate predicted means (±1 SE); numbers above bars indicate sample sizes.
Discussion
There is increasing evidence that conditions experienced before birth can have profound effects on the subsequent growth, survival, and reproductive capacity of individuals (1–8). For example, the prenatal acquisition of hormones from developing neighbors in viviparous animals has been shown to have significant consequences for adult morphology, physiology, and behavior both in mammals (9, 12), including humans (11, 13–20), as well as in viviparous lizards (29). An intriguing implication of such effects is that if, through sex-specific interactions among offspring, litter sex ratios influence offspring success in adulthood, this interaction will modify optimal offspring sex ratios for mothers (10). However, so far, the fitness consequences of prebirth sex ratio for offspring and mothers are largely unknown for viviparous animals, and hence their fitness consequences for the mother cannot be incorporated into models of adaptive sex-ratio bias (10). Our study shows that human females who had a male co-twin have reduced lifetime reproductive success compared to females who had a same-sex co-twin. This effect arises because a female who had a male co-twin has both reduced probability of marrying and reduced fecundity compared to females born with a female co-twin. Moreover, our results show that, as a consequence of these effects, mothers who produced opposite-sex twins have fewer grandchildren (and hence lower evolutionary fitness) than mothers who produced same-sex twins. These results provide the first evidence that sex-ratio adjustment influences offspring fitness because of sex-specific interactions between offspring prebirth, and have significant implications for understanding the adaptive nature of sex ratios in humans and other viviparous animals.
Our results could be confounded by two sources of variation, but we found little supporting evidence. First, our results are unlikely to be confounded by social or life-history differences between mothers producing male–male, male–female, or female–female twins. We found no evidence to suggest that the combination of twins produced by mothers is influenced by social class (χ2 = 1.55, P = 0.46) or their age, number of previous children produced and raised, or any other life-history character (30). Furthermore, males from male–female or male–male twin births, or females from male–female and female–female twin births, do not differ in their own social class in adulthood (males: χ2 = 0.20, P = 0.65; females: χ2 = 0.61, P = 0.43), which could have independent effects on their reproductive success. Finally, all our analyses also control for any significant effects (where applicable) of birth order, maternal age at birth, number of other competing siblings, whether both or just one of the twins was raised to adulthood, as well as geographic (area) and temporal (birth cohort) variation in fertility and mortality (22, 23). In addition, we did not find a significant interaction between twin sex ratio and birth population, suggesting that our results were common to all five populations studied.
Second, although our results provide strong support for the hypothesis that testosterone from male co-twins is associated with an impairment of female-twin fitness, they are also consistent with the alternative hypotheses that either (i) females with “more dominant” male co-twins may be simply outcompeted nutritionally before or after birth, or (ii) females may become masculinized after birth or suffer from social competition because of growing up with a similar-aged brother (11). If daughters are less favored than sons by parents, being born with a brother may be particularly detrimental to females because of reduced parental support. This latter effect could arise if sons are more valuable than daughters or if daughters are more likely to be required to forego marriage to help at home when they have a male co-twin. Finally, it is possible that sibling interactions after birth could generate sex-ratio effects on offspring fitness without direct competition for resources. For example, in humans, the likelihood of getting a viral infection and its severity are up to twice as high if transmitted from a sibling of the opposite sex than from one of the same sex (31, 32).
None of the above alternatives is supported by our results. If females with a male co-twin are outcompeted nutritionally or more susceptible to disease, we would expect higher female mortality in the presence of a male co-twin than in the presence of a female co-twin. We found no evidence for this: Females with male versus female co-twins have similar survival to adulthood (see Results). Additionally, we would expect the impairment of female success to be reduced in situations of high resource availability. Again, we found no evidence for this because there is no suggestion that either high social class (landowners) or favorable geographic location (food-predictable island areas) influences the difference in success between females with a male versus female co-twin (all interaction terms with social class and geographic location were nonsignificant; P > 0.1). The lack of a geographic effect is particularly important because we have shown previously that twins have lower mortality in food-predictable island populations compared to unpredictable inland areas (22, 30).
If the differences were caused by postnatal masculinization or social-preference effects, we would expect that females would do better if their male co-twin dies shortly after birth and the females are raised after birth as singletons. In contrast, we found no evidence to suggest that daughter success improves if their male co-twin dies shortly (within 3 months) after birth. Females born with a male co-twin but raised as singletons after birth still have impaired lifetime fecundity and lifetime reproductive success compared to females born with a female co-twin but raised as singletons. This result makes it difficult to interpret our findings as after-birth social or parental-preference effects. This point is further emphasized by the fact that we failed to detect a significant interaction between social class and success of females with a male co-twin: In many societies, females can be preferred over males among lower classes, whereas males can be preferred among the upper classes (33). Finally, that the death of a male co-twin shortly after birth fails to improve female success also provides further support for the idea that reduced female success is not because of females simply being outcompeted by male co-twins nutritionally because the greatest nutritional demand of growing infants is not during gestation, but during late lactation (34).
In conclusion, we have found that females with male co-twins are significantly disadvantaged compared to those with female co-twins or compared to males with female or male co-twins. These results are difficult to explain by postnatal effects (nutritional, social, or other) because our results remain the same when individuals are gestated with co-twins but reared as singletons (because of co-twin death within 3 months after birth). Studies from other viviparous animals show convincingly that sex hormones can diffuse through the fetal membranes and amniotic fluid and that the acquisition of testosterone from developing male neighbors can have substantial consequences for female morphology, physiology, and behavior (9, 12); previous evidence from humans is also consistent with these observations (11–20). Hence, the most likely mechanism for our observed reduction of female-twin fitness is the acquisition of testosterone from the developing male co-twin. Notwithstanding, our results show that being exposed to a male co-twin in utero has two major effects on females. First, it reduces their marriage probability, possibly because females who have a male co-twin can have masculinized sexually dimorphic anatomical traits (16–20) and attitudes/behaviors (11). Studies in humans and other mammals show that the more feminine females are preferred as mates by males (9, 35, 36), and direct evidence from laboratory animals confirms that intrauterine position can affect female attractiveness to males (37, 38). Second, exposure to male co-twins reduces female fecundity, possibly because elevated levels of testosterone during development promote the onset of diseases that compromise fertility, such as polycystic ovary syndrome (39, 40) and reproductive cancers (41, 42).
Finally, that gestation sex ratios can have significant effects on offspring (and consequently maternal) fitness, because of hormonal interactions among fetuses, have important implications for optimal sex allocation in mammals generally, as well as estimations of monozygotic versus dizygotic twinning rates in humans. Although previous studies suggest that chromosomal sex determination constrains adaptive sex-ratio adjustments in mammals (43, 44), recent studies show that such adjustments in sex ratios are possible, but are only adaptive when clear and predictable relationships between sex-ratio biases and fitness occur (10, 45). In this study, sex-specific interactions among offspring led to mothers who produced opposite-sex twins having 19% fewer grandchildren than mothers who produced same-sex twins. Therefore, the maternal sex ratio can directly influence offspring fitness because of sex-specific interactions among offspring in utero, and this interaction could affect optimal sex ratios for mothers. Our results suggest that viviparous animals that deliver more than one offspring per litter may therefore represent a particularly rich testing ground for adaptive sex-ratio biases in species with chromosomal sex determination. Second, rates of nonheritable (monozygotic) versus heritable (dizygotic) twinning from demographic data are commonly calculated by using the Weinberg's rule based on the ratio of same versus opposite-sex twinning in a population (46). The results of this study show that sex-ratio biases toward same-sex twins would be predictably adaptive in humans, leading to the possibility that such calculations could lead to underestimations of the levels, and hence the adaptive nature, of heritable twinning in humans.
Materials and Methods
Study Population.
The consequences of having a same- versus opposite-sex co-twin for lifetime reproductive success of males and females were studied by using demographic data collected from Finnish population registers of the preindustrial era. The Lutheran Church has kept birth/baptism, marriage, and death/burial registers of each parish in the country since the 17th century, covering the whole population of Finland from 1749 onward. Using these registers, it is possible to follow the reproductive and marital details of each individual from birth to death because the whole population practiced the Lutheran religion, and everybody who died (in most cases including stillborn and infants who died before baptism) were buried in a cemetery and recorded in the book of deaths (47). Information on the rates of abortion and infanticide in these populations is not known, but (active) infanticide is considered rare because it was highly punishable by the society (47), and there are only a few recorded incidences of infanticide occurring in the study parishes during the study era.
The data were collected by using church records from five geographically separate rural archipelago and mainland parishes (Hiittinen, 60°N 22°30′E; Ikaalinen, 61°45′N 23°0E; Kustavi, 60°30′N 21°30′E; Pulkkila, 64°15′N 26°E; and Rymättylä, 60°15′N 22°E) during the 18th and 19th centuries (22, 23, 30). The study era ended before industrialism, more liberal economics, and improvements in health care were likely to have had significant effects on survivorship and the standard of living in Finland, and before any of the modern birth control methods were available to limit fertility (48).
All twins born in the study parishes during 1734–1888 were traced from the church registers (n = 377 twin births). Of these, 105 were male–male, 117 were female–female, and 155 were male–female twin births. The vast majority of the twins in the sample were likely to be dizygotic; the estimated dizygotic and monozygotic twinning rates in Finnish archipelago during 1653–1949 were 1.64% and 0.28%, respectively (49), although these dizygotic twinning rates may represent an underestimate (see Discussion). Each twin was followed from birth to death or at least until they were 50 years old and known to have ceased reproducing. Of all of the twins, 35% survived to adulthood (age 15 years) and 16% reproduced in their lifetimes. We recorded all marriages and the birth, gender, and survival of all the children produced by each. Information on the occupation of each man (or, for women, the occupations of their husbands) allowed us to rank the social class of each adult twin and also the social class (landowners vs. landless) of their parents, which is a correlate of resources available (23).
Statistical Analyses.
The effects of having a same-sex versus an opposite-sex co-twin for female and male marriage and reproductive probability were analyzed by logistic regressions with binomial error structure and logit link function. Effects on their lifetime numbers of children born (fecundity) and numbers raised to age 15 years were analyzed by using GLMs with normal error structures. Differences in the numbers of grandchildren born to mothers delivering same versus opposite-sex twins were analyzed by using GLM. Residuals of all GLM models were normally distributed and variances were homogeneous (Levene's test, P > 0.05). In each model, personal or parental social class, birth order, maternal age at birth, number of competing siblings, whether both or just one of the twins was raised to adulthood, and geographic (area) and temporal (birth cohort) variation in fertility and mortality were fitted and included in the final model, if significant, to control for potentially confounding variables.
The effects of having a same- versus opposite-sex co-twin for female and male survivorship to age 15 years were analyzed by using Cox proportional hazards regression and estimated by the Kaplan–Meier method (50, 51). Assumption of proportional hazards in Cox regression was investigated by including the time-dependent covariates of independent variables in the model. If a time-dependent effect of explanatory variable was found (indicating nonproportional hazards), the time-dependent covariate was included in the final model (51). The analyses controlled for effects of maternal age, social class, family size, birth cohort, and geographic differences on child survival.
Because virtually all mothers produced just one set of twins in their lifetime, and there were only 15 pairs of twins of 377 for which both individuals reproduced (nine female–female, four male–female, and two male–male), correlated measures from the same mother or twin delivery were likely to be of negligible influence for the analyses of reproductive success. Removing such data points from the analyses did not change the results. All analyses were conducted with SAS statistical package version 9.1 (SAS Institute, Cary, NC).
Acknowledgments
We thank Terho Koira for continuing support; Kimmo Pokkinen, Aino Siitonen, and Timo Verho for collecting the data; and Charlotte Faurie, Ben Hatchwell, Stewart Plaistow, Ian Rickard, the editor, and three anonymous referees for helpful comments on the manuscript. This work was supported by the Royal Society, United Kingdom (V.L. and A.F.R.), the Academy of Finland (V.L. and J.E.P.), and the Finnish Cultural Foundation (J.E.P.).
Abbreviation
- GLM
general linear model.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lummaa V, Clutton-Brock TH. Trends Ecol Evol. 2002;17:141–147. [Google Scholar]
- 2.Lindström J. Trends Ecol Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- 3.Weber GW, Prossinger H, Seidler H. Nature. 1998;391:754–755. doi: 10.1038/35781. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJP. Mothers, Babies and Disease in Later Life. London: BMJ Publishing Group; 1994. [Google Scholar]
- 5.Doblhammer G, Vaupel JW. Proc Natl Acad Sci USA. 2001;98:2934–2939. doi: 10.1073/pnas.041431898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore SE, Cole TJ, Poskitt EME, Sonko BJ, Whitehead RG, McGregor IA, Prentice AM. Nature. 1997;388:434. doi: 10.1038/41245. [DOI] [PubMed] [Google Scholar]
- 7.Lummaa V, Tremblay M. Proc R Soc London B. 2003;270:2355–2361. doi: 10.1098/rspb.2003.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber S, Fieder M, Wallner B, Iber K, Moser G. Hum Reprod. 2004;19:1081–1082. doi: 10.1093/humrep/deh247. [DOI] [PubMed] [Google Scholar]
- 9.Ryan BC, Vandenbergh JG. Neurosci Biobehav Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 10.Uller T. Biol Rev. 2006;81:207–217. doi: 10.1017/S1464793105006962. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Even MD, Dhar MG, vom Saal FS. J Repod Fert. 1992;96:709–716. doi: 10.1530/jrf.0.0960709. [DOI] [PubMed] [Google Scholar]
- 13.Miller EM. Pers Indiv Diff. 1994;17:511–529. [Google Scholar]
- 14.Blumrosen E, Goldman RD, Blickstein I. J Perinatal Med. 2002;30:510–513. doi: 10.1515/JPM.2002.079. [DOI] [PubMed] [Google Scholar]
- 15.Glinianaia S, Magnus P, Harris JR, Tambs K. Int J Epidemiol. 1998;27:657–659. doi: 10.1093/ije/27.4.657. [DOI] [PubMed] [Google Scholar]
- 16.van Anders SM, Vernon PA, Wilbur CJ. Horm Behav. 2006;49:315–319. doi: 10.1016/j.yhbeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 17.McFadden DA. Proc Natl Acad Sci USA. 1993;90:11900–11904. doi: 10.1073/pnas.90.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boklage CE. Am J Hum Genet. 1985;37:591–605. [PMC free article] [PubMed] [Google Scholar]
- 19.Miller EM. Opt Vis Sci. 1995;72:34–36. doi: 10.1097/00006324-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Dempsey PJ, Townsend GC, Richards LC. Am J Hum Biol. 1999;11:577–586. doi: 10.1002/(SICI)1520-6300(199909/10)11:5<577::AID-AJHB1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.vom Saal F, Moyer C. Biol Reprod. 1985;32:1116–1126. doi: 10.1095/biolreprod32.5.1116. [DOI] [PubMed] [Google Scholar]
- 22.Lummaa V, Haukioja E, Lemmetyinen R, Pikkola M. Nature. 1998;394:533–534. doi: 10.1038/28977. [DOI] [PubMed] [Google Scholar]
- 23.Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell AF. Nature. 2004;428:178–181. doi: 10.1038/nature02367. [DOI] [PubMed] [Google Scholar]
- 24.vom Saal F. J Anim Sci. 1989;67:1824–1840. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- 25.Kaprio J, Rimpelä A, Viken RJ, Winter T, Rimpelä M, Rose RJ. Hum Biol. 1995;67:739–753. [PubMed] [Google Scholar]
- 26.Rose RJ, Kaprio J, Winter T, Dick DM, Viken RJ, Pulkkinen L, Koskenvuo M. Psychol Sci. 2002;13:263–267. doi: 10.1111/1467-9280.00448. [DOI] [PubMed] [Google Scholar]
- 27.Christensen K, Basso O, Kyvik KO, Juul S, Boldsen J, Vaupel JW, Olsen J. Epidemiology. 1998;9:189–192. [PubMed] [Google Scholar]
- 28.Loehlin JC, Martin NG. Behav Genet. 1998;28:21–27. doi: 10.1023/a:1021452630561. [DOI] [PubMed] [Google Scholar]
- 29.Uller T, Massot M, Richard M, Lecomte J, Clobert J. Evolution (Lawrence, Kans) 2004;58:2511–2516. doi: 10.1111/j.0014-3820.2004.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 30.Lummaa V, Jokela J, Haukioja E. J Anim Ecol. 2001;70:739–746. [Google Scholar]
- 31.Aaby P. Lancet. 1992;340:388–391. doi: 10.1016/0140-6736(92)91470-s. [DOI] [PubMed] [Google Scholar]
- 32.Pison G, Aaby P, Knudsen K. Br Med J. 1992;304:284–289. doi: 10.1136/bmj.304.6822.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bereczkei T, Dunbar RIM. Proc R Soc London B. 1997;264:17–22. [Google Scholar]
- 34.Clutton-Brock TH, Albon SD, Guinness FE. Nature. 1989;337:260–262. doi: 10.1038/337260a0. [DOI] [PubMed] [Google Scholar]
- 35.Saino N, Romano M, Innocenti P. Behav Ecol Sociobiol. 2006;60:447–454. [Google Scholar]
- 36.Barrett B, Dunbar R, Lycett J. Human Evolutionary Psychology. Basingstoke, UK: Palgrave; 2002. [Google Scholar]
- 37.vom Saal F, Bronson F. Biol Reprod. 1978;19:842–853. doi: 10.1095/biolreprod19.4.842. [DOI] [PubMed] [Google Scholar]
- 38.Rines J, vom Saal F. Horm Behav. 1984;18:117–129. doi: 10.1016/0018-506x(84)90037-0. [DOI] [PubMed] [Google Scholar]
- 39.Abbott DH, Padmanabhan V, Dumesic DA. Rep Biol Endocrinol. 2006;4:17. doi: 10.1186/1477-7827-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumesic DA, Schramm RD, Abbott DH. Rep Fert Develop. 2005;17:349–360. doi: 10.1071/rd04092. [DOI] [PubMed] [Google Scholar]
- 41.Potischman N, Troisi R, Thadhani R, Hoover RN, Dodd K, Davis WW, Sluss PM, Hsieh CC, Ballard-Barbash R. Cancer Epidemiol Biomarkers Prev. 2005;14:1514–1520. doi: 10.1158/1055-9965.EPI-04-0869. [DOI] [PubMed] [Google Scholar]
- 42.Luke B, Hediger M, Min SJ, Brown MB, Misiunas RB, Gonzalez-Quintero VH, Nugent C, Witter FR, Newman RB, Hankins GDV, et al. Paediat Perinatal Epidemiol. 2005;19:41–47. doi: 10.1111/j.1365-3016.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton WD. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 44.Clutton-Brock TH, Iason GR. Q Rev Biol. 1986;61:339–374. doi: 10.1086/415033. [DOI] [PubMed] [Google Scholar]
- 45.West SA, Sheldon BC. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- 46.Bulmer MG. The Biology of Twinning in Man. Oxford: Clarendon; 1970. [Google Scholar]
- 47.Gille H. Pop Studies. 1949;3:3–65. [Google Scholar]
- 48.Soininen AM. Old Traditional Agriculture in Finland in the 18th and 19th Centuries. Forssa, Finland: Forssan Kirjapaino Oy; 1974. [Google Scholar]
- 49.Eriksson AW. Human Twinning in and around the Åland Islands. Helsinki: Societas Scientiarum Fennica; 1973. [Google Scholar]
- 50.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Inst; 1995. [Google Scholar]
- 51.Collett D. Modelling Survival Data in Medical Research. 2nd Ed. Boca Raton, FL: Chapman & Hall/CRC; 2003. [Google Scholar]