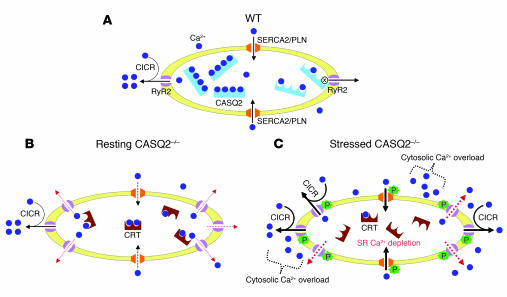
Figure 6. A model for CPVT based on abnormal SR Ca2+ homeostasis in CASQ2-deficient myocytes.
(A) Under resting conditions, SR Ca2+ (blue circles) is plentiful and buffered by CASQ2 (blue rectangles) in WT myocyte. Ca2+ influx via L-type Ca2+ channel (left side) causes Ca2+-induced Ca2+ release (CICR) through cardiac RyR2 channels (purple). Reuptake of cytosolic Ca2+ (black arrows) occurs via the SERCA/PLN complex (orange). When SR Ca2+ concentration falls (right half), CASQ2 binds to the RyR2 channel, closing the channel (x), thus preventing Ca2+ leak (right side arrow). (B) In CASQ2-deficient myocytes, CRT (brown symbols) replaces CASQ2. Total SR Ca2+ is lower than in WT myocytes due to lower Ca2+-binding capacity by CRT (50% of CASQ2) and diastolic Ca2+ leak (red dashed arrows) through abundant RyR2 channels and inadequate calstabin. SERCA2-mediated Ca2+ reuptake may also be impaired (dashed black arrows) due to increased free Ca2+ gradient given lower Ca2+-binding capacity of CRT. Total SR Ca2+ transients and cytosolic Ca2+ are near normal. (C) Catecholaminergic stress applied to CASQ2-deficient myocytes phosphorylates RyR2 channels and PLN, dissociating calstabin from RyR2. Excessive RyR2 activity causes extensive diastolic Ca2+ leak, further depletes SR Ca2+, reduces SR Ca2+ transients, and increases cytosolic Ca2+ levels. Excess cytosolic Ca2+, effluxed via the Na+/Ca2+ exchanger, increases Na+ entry into myocyte, which increases the probability of delayed after-polarization and cardiac arrhythmia. Toxicity of Ca2+ overload may increase myocyte death.