Abstract
The genetic and epigenetic events underlying cutaneous squamous cell carcinoma (SCC) have been actively studied; however, no resulting preventative or therapeutic strategies have successfully targeted this lesion, apart from surgery. In this issue of the JCI, two novel regulators of SCC pathogenesis are introduced, gain-of-function mutations in the p53 gene, reported by Caulin et al., and chemokine sequestration by the D6 receptor, reported by Nibbs et al. (see the related articles beginning on pages 1884 and 1893, respectively). These studies provide new twists and insights into the development of this potentially lethal disease.
Nonmelanoma skin cancer (NMSC) is the most common type of human malignancy, with over one million new cases in the United States annually (1–3). NMSC includes basal cell carcinoma and squamous cell carcinoma (SCC), with SCCs constituting approximately 20% of all NMSCs. In contrast to basal cell carcinomas, SCCs characteristically exhibit a high propensity for invasion and metastasis and may be lethal (1, 2). While the genetic and epigenetic events associated with SCC pathogenesis have been extensively studied, no resulting therapeutic approaches have been generated for the prevention or treatment of this potentially lethal disease. This, coupled with the escalating incidence of NMSC over the last few decades, has made this disease a major public health issue.
The p53 oncogene: implications for cutaneous SCC
It is widely accepted that genetic insults are indispensable in the formation of a frank tumor. In the development of human cutaneous SCCs, alterations in ras genes (10%–30% incidence) (3, 4) and the p53 tumor suppressor gene (40%–50% incidence) (5, 6) have been most heavily implicated. While the majority of these lesions are missense mutations, the functional assessment of p53 missense mutations is complicated in that some give rise to a loss-of-function or null phenotype classically associated with tumor suppressor genes, whereas the majority of p53 missense mutations appear to result in a gain-of-function phenotype. While the presence of both types of p53 mutations in SCCs denotes a selection advantage to these genetic lesions, whether gain-of-function or loss-of-function mutations are more critical for SCC development is unclear. Therefore, the understanding of p53 phenotype status, i.e., tumor suppressive versus oncogenic, as it relates to SCC formation and progression is of paramount importance for the development of relevant therapeutic approaches that target this gene.
In this issue of the JCI, Caulin and coworkers (7) address this problem with an elegant transgenic mouse skin model that delivers allelic doses of oncogenic K-ras (8) in combination with either p53 gain-of-function or null mutations under the regulatory control of an inducible Cre recombinase and targeted to the proliferative layer of the epidermis by the cytokeratin K5 promoter. Using this approach, the authors clearly show that certain p53 missense mutations occurring in human SCCs demonstrate gain-of-function properties in vivo and that p53 gain-of-function mutations accelerate both the frequency and progression of SCCs genetically initiated with oncogenic ras. For over a decade, the conventional regulation of cutaneous SCC by p53 tumor suppressor function was based on the observation that reductions in p53 typically do not increase the frequency or incidence of tumor formation but rather increase the frequency of malignant conversion to SCC (9). The current study confirms this traditional role for loss-of-function p53 mutations in malignant conversion to SCC and also provides completely novel roles for gain-of-function mutant p53 in the early stages of benign papilloma formation and during late-stage metastasis (Figure 1). What is most striking about this study is the accelerated malignant progression, genomic instability, and incidence of metastasis in cells harboring p53 gain-of-function mutations versus p53-null cells. These results indicate that p53 has potent oncogenic properties, and in addition to the p53 mutation spectra that result in the null phenotype critical for conversion to SCC, Caulin et al. have now demonstrated that p53 gain-of-function mutations may actually shift the epigenetic environment of an SCC cell toward that of a highly malignant spindle cell phenotype. Spindle cell tumors, while rarely observed in mouse skin carcinogenesis models, represent the most advanced stage of SCC, are composed almost entirely of undifferentiated cells, and characteristically exhibit a highly aggressive and invasive behavior. Since p53 gain-of-function mutants are transcriptionally active in vitro (10), further studies are needed to identify the potential therapeutic targets within the unique mRNA pool that is maintained by these gain-of-function mutants in vivo. As noted by the authors, a relatively high number of cells harboring p53 missense mutations exist in sun-exposed human epidermis but ultimately never result in SCC formation (6). This supports the multi-hit carcinogenesis model and the hypothesis that additional genetic alterations are required for the development of SCC. However, future studies that allow for more precise targeting of p53 gain-of-function mutations in the epidermis may also illustrate the importance of target cell phenotype, i.e., stem cell versus progenitor or transit amplifying cell, in p53-driven SCC formation.
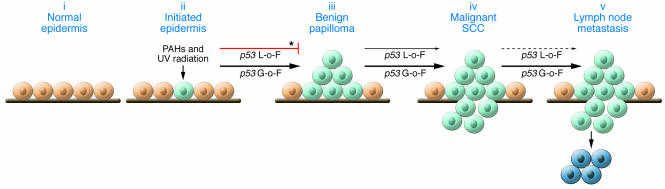
Figure 1. Impact of p53 gain-of-function versus null mutations in epidermal carcinogenesis.
In this issue of the JCI, Caulin et al. (7) compare the impact of p53 gain-of-function (G-o-F) versus loss-of-function (L-o-F) mutations in multistage skin carcinogenesis progressing from normal epidermis (i) to initiated epidermis (ii), benign papillomas (iii), malignant SCC (iv), and finally lymph node metastasis (v). p53 gain-of-function mutations effectively stimulate early and late stages of epidermal carcinogenesis, whereas null mutations primarily influence malignant conversion to SCC. Light blue cells, initiated cells containing ras and/or p53 mutations; dark blue cells, metastatic cells; thin arrow, inducer; bold arrow, strong inducer; dotted arrow, little to no effect; asterisk, data previously demonstrated by Kemp et al. (9). PAH, polycyclic aromatic hydrocarbon.
D6: an atypical chemokine receptor
In general, the relationship between inflammation and cancer has been well documented, from the influence of chronic inflammatory agents on neoplastic cell proliferation in founding a tumor to later stages of tumor progression associated with immune suppression, and clearly indicates that both innate and adaptive responses of the immune system are deeply rooted in cancer cell growth, progression, and migration (11).
A link between skin inflammation and SCC formation was reported as early as 1828 as Marjolin ulcer (12), where SCC development was observed at sites of chronic skin inflammation induced by thermal injury. More recently, the association between SCC development and inflammation has been extended to several human skin pathologies associated with ulceration and further substantiated in a number of transgenic murine skin carcinogenesis models (see ref. 13 for an excellent review), collectively indicating that chronic inflammatory stimuli may be a driving force for epidermal neoplastic cell progression. Despite these efforts, very few studies have surfaced that identify specific inflammatory molecules that are required for de novo skin carcinogenesis (14–16).
In this issue of the JCI, Nibbs and coworkers (17) have assessed the requirement of the atypical chemokine receptor D6 in chemically induced skin inflammation and SCC formation. Previously, this group and others have shown that D6 regulates skin inflammatory responses by reducing the bioavailability of inflammatory chemokines (18), and in the present study they postulate that the sequestration of inflammatory chemokines by D6 is essential to suppressing epidermal carcinogenesis. To support their hypothesis, they show that D6-deficient mice are acutely sensitive to chemically induced SCC formation and in doing so provide the first in vivo evidence that chemokine sequestration is essential for suppressing cutaneous SCC (17). The phenotypic response of D6-deficient mice to acute treatment with the irritant phorbol ester is consistent with the reported role for this receptor in the regulation of heightened inflammatory chemokine levels (18). Their findings in experimental mouse skin carcinogenesis were corroborated by the induction of D6 in lymphatic endothelial cells in close proximity to invasive SCC cells from human oral SCCs. In their model, production of the proinflammatory CC chemokine ligand 3 (CCL3) is prolonged in D6-deficient skin after treatment with phorbol ester, leading to chronic recruitment of two cell types known to be involved in cutaneous SCCs, CD3+ T cells and dermal mast cells (Figure 2). In the absence of D6 chemokine sequestration, the exacerbation of the proinflammatory epidermal microenvironment sensitizes the epidermis to hyperproliferation and ras-driven tumor formation (Figure 2). Finally, the persistence of the proinflammatory chemokine response maintains chronic recruitment of lymphocytes and mast cells and stimulates SCC invasion and metastasis (Figure 2). According to this model, the ability of a particular inflammatory agent to overcome the threshold of normal D6-mediated CCL3 interception should directly correlate with tumor-promoting capability.
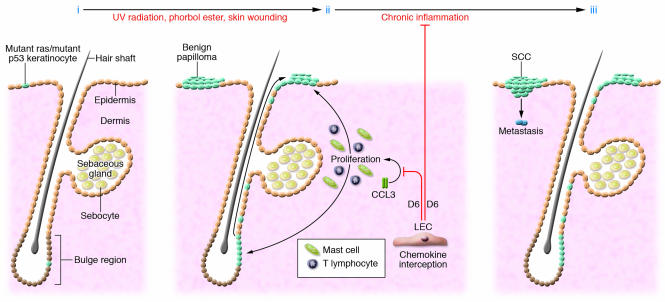
Figure 2. D6-mediated chemokine sequestration as a threshold barrier for cutaneous SCC.
(i) Basal cells in the interfollicular epidermis and hair follicles sustain mutations (light blue cells) due to exposure to UV radiation, chemical carcinogens, viruses, and other pathogens; however, without epigenetic intervention, most of these mutations will lay dormant. (ii) Repeated skin irritation or wounding can heighten inflammatory chemokine levels over that which can be sustained through interception by the D6 receptor (expressed by lymphatic endothelial cells [LECs] in the dermis), resulting in the exacerbated levels of mast cells and T lymphocytes critical for stimulating benign papilloma formation. (iii) In the chronic situation, high levels of immune cells are maintained in the skin and enhance malignant conversion and metastasis of neoplastic epidermal cells (dark blue).
Are p53 and inflammatory chemokines linked?
Collectively, these studies (7, 17) give critical insight into two apparently distinct therapeutic approaches to potentially target cutaneous SCC. However, the induction of late-stage metastases common to both p53 and D6 transgenic mouse models is normally a rare event in experimental mouse skin carcinogenesis and therefore raises the question as to whether there is a more direct relationship between certain proinflammatory signals and p53 gain-of-function mutations as they relate to SCC pathogenesis. For example, can a heightened inflammatory chemokine response critically influence the p53 status in otherwise normal epidermal cells and/or pre-neoplastic epidermal cells that have sustained additional genetic insults? This concept fits well with the UV carcinogenesis model in human skin, in which small clusters of p53 mutant cells exist prior to actinic keratosis (AK) and SCC development (6) and may be selected for clonal expansion by a chronic inflammatory stimulus (13). In addition, AK are reported to undergo a short inflammatory phase just prior to conversion to SCC that is accompanied by an increase in p53 levels (19). If a heightened inflammatory state in the skin can influence p53 gain-of-function status, this may have important implications for the prognosis of cutaneous SCCs associated with skin inflammatory pathologies. Although the precursor to chemically induced murine SCCs, squamous papilloma, does not typically harbor p53 mutations, increased levels of wild-type p53 are observed (20). A better understanding of the impact of D6-mediated chemokine sequestration on UV-induced carcinogenesis may help to address this concept.
Footnotes
Nonstandard abbreviations used: NMSC, nonmelanoma skin cancer; SCC, squamous cell carcinoma.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:1752–1755 (2007). doi:10.1172/JCI32719.
See the related article beginning on page 1884.
See the related article beginning on page 1893.
References
- 1.Murad A., Ratner D. Primary care: cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 2.Albert M.R., Weinstock M.A. Keratinocyte carcinoma. CA Cancer J. Clin. 2003;53:292–302. doi: 10.3322/canjclin.53.5.292. [DOI] [PubMed] [Google Scholar]
- 3.Pierceall W.E., Goldberg L.H., Tainsky M.A., Mukhopadhyay T., Ananthaswamy H.N. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol. Carcinog. 1991;4:196–202. doi: 10.1002/mc.2940040306. [DOI] [PubMed] [Google Scholar]
- 4.Spencer J.M., Kahn S.M., Jiang W., DeLeo V.A., Weinstein I.B. Activated ras genes occur in human actinic keratoses, premalignant precursors to squamous cell carcinomas. Arch. Dermatol. 1995;131:796–800. [PubMed] [Google Scholar]
- 5.Bolshakov S., et al. p53 mutations in human aggressive and nonaggresive basal and squamous cell carcinomas. Clin. Cancer Res. 2003;9:228–234. [PubMed] [Google Scholar]
- 6.Brash D.E., et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caulin C., et al. An inducible mouse model for skin cancer reveals distinct roles for gain- and loss-of-function p53 mutations. . J. Clin. Invest. 2007;117:1893–1901. doi: 10.1172/JCI31721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson E.L., et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemp C.J., Donehower L.A., Bradley A., Balmain A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell. 1993;74:813–822. doi: 10.1016/0092-8674(93)90461-x. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer D., et al. Gain of function mutations in p53. Nat. Genet. 1993;4:42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- 11.Coussens L.M., Werb Z.2002Inflammation and cancer . Nature. 420860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marjolin, J.N. 1828. Ulcère [In French]. In Dictionnaire de médecine. Paris Béchet jeune. Paris, France. 31–50. [Google Scholar]
- 13.Mueller M.M. Inflammation in epithelial skin tumours: old stories and new ideas. Eur. J. Cancer. 2006;42:735–744. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Moore R.J., et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 15.Coussens L.M., Tinkle C.L., Hanahan D., Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girardi M., et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 17.Nibbs R.J.B., et al. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J. Clin. Invest. 2007;117:1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson T., et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat. Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 19.Berhane T., Halliday G.M., Cooke B., Barnetson R.S. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br. J. Dermatol. 2002;146:810–815. doi: 10.1046/j.1365-2133.2002.04720.x. [DOI] [PubMed] [Google Scholar]
- 20.Owens D.M., Wei S.-J.C., Smart R.C. A multihit, multistage model of chemical carcinogenesis. Carcinogenesis. 1999;20:1837–1844. doi: 10.1093/carcin/20.9.1837. [DOI] [PubMed] [Google Scholar]