Table 1.
Inhibitory activities of the adamantane-based ureas 1{1}, 2{1}, 3{1}, and 4{1} derived from the amines 1 to 4
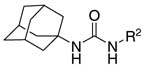
| Compounda | R2-NH2 | IC50b(nM) | % inhibitionc |
|---|---|---|---|
| 1{1} | 1 | 0.5 | 86 ± 9 |
| 2{1} | 2 | 0.5 | 75 ± 12 |
| 3{1} | 3 | 30 | 19 ± 7 |
| 4{1} | 4 | 100 | 10 ± 3 |
The notation for the compound number: amine number {isocyanate number}, for example, 2{1} indicates a compound made by the combination of amine 2 with isocyanate 1.
As determined via a kinetic fluorescent assay.10
Determined via an end-point fluorescent assay, results are means ± SD of three separate experiments.11
