Figure 8.
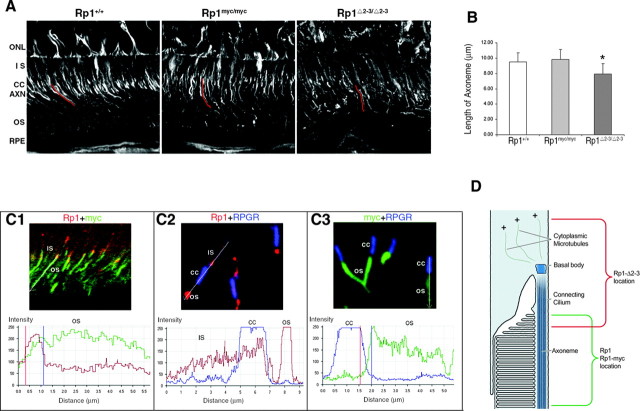
The DCX domains in the Rp1 protein are active in vivo. A, Frozen sections of retina from mice with the genotypes indicated were stained with antibodies to acetylated α-tubulin and viewed by confocal microscopy. Individual axonemes were identified by viewing the confocal images at high magnification. The axonemes were traced (example traces in red), and the cord lengths of 50 axonemes from each type of mouse were then measured using the LSM510 Meta software. ONL, Outer nuclear layer; IS, inner segment; CC, connecting cilium; AXN, axoneme; OS, outer segment; RPE, retinal pigment epithelium. B, The mean lengths ± SD of the 50 axonemes from each genotype of mouse are indicated. The length of the axonemes in the RpΔ2-3/Δ2-3 mice is significantly shorter than those of the wild-type and Rp1-myc mice (*p < 0.01). C, Frozen sections of the retinas from 2-week-old double mutant Rp1myc/Δ2-3 mice were coimmunostained with pairs of antibodies to detect the Rp1-Δ2-3 protein (anti-C-Rp1, red), the Rp1-myc protein (anti-myc, green), and the connecting cilium (anti-RPGR, blue). The merged images and quantitative analyses are shown for each antibody pair. The analysis profiles start proximally and proceed distally into the outer segment. The Rp1-myc protein is localized correctly to the outer segment portion of the axoneme (C1, C3). In contrast, the Rp1-Δ2-3 protein is mislocalized into the connecting cilium and inner segment (C1, C2). D, Diagram of the junction between the inner and outer segments of a rod photoreceptor cell, showing the location of the wild-type Rp1 and Rp1-myc proteins (green) and the Rp1-Δ2-3 deletion protein (red).