Abstract
Angiotensin II (Ang II)–induced arterial baroreflex dysfunction is associated with superoxide generation in the brain. Exercise training (EX) improves baroreflex function and decreases oxidative stress in cardiovascular diseases linked to elevated central Ang II. The aim of this study was to determine whether previous EX prevents baroreflex impairment caused by central administration of exogenous Ang II via an Ang II–superoxide mechanism. Four groups of rats were used: non-EX artificial cerebrospinal fluid infused, non-EX Ang II infused, EX artificial cerebrospinal fluid infused, and EX Ang II infused. Rats were treadmill trained for 3 to 4 weeks and subjected to intracerebroventricular infusion of Ang II over the last 3 days of EX. Twenty-four hours after the end of EX, the arterial baroreflex was assessed in anesthetized rats. Compared with non-EX artificial cerebrospinal fluid–infused rats, Ang II significantly decreased baroreflex sensitivity (maximum gain: 3.0 ± 0.2% of maximum per millimeter of mercury versus 1.6 ± 0.1% of maximum per millimeter of mercury; P < 0.01), which was abolished by acute intracerebroventricular infusion of the Ang II type 1 receptor antagonist losartan and the reduced nicotinamide-adenine dinucleotide phosphate oxidase inhibitor apocynin. EX prevented the decrease in baroreflex sensitivity and downregulated Ang II type 1 receptor and NADPH oxidase subunit protein expression in the paraventricular nucleus of Ang II–infused rats. Finally, EX decreased superoxide production in the paraventricular nucleus of Ang II–infused rats. These results indicate that EX improves arterial baroreflex function in conditions of high brain Ang II, which is mediated by the central Ang II type 1 receptor and associated with a reduction in central oxidative stress.
Keywords: exercise, baroreflex, sympathetic nerve activity, reactive oxygen species, AT1 receptor
Impairment of arterial baroreflex function is an important feature in cardiovascular diseases, such as chronic heart failure (CHF) and hypertension.1,2 This abnormality increases cardiovascular risk.3 Recent studies indicate that the blunted arterial baroreflex is related to an enhanced central angiotensin II (Ang II) mechanism, because blockade of Ang II type 1 receptors (AT1Rs) in the brain restored baroreflex sensitivity.4 Intracerebroventricular (icv) infusion of Ang II depresses arterial baroreflex function in normal animals.5 However, specific brain nuclei and the intracellular mechanism for Ang II–induced baroreflex dysfunction have not been fully identified. The paraventricular nucleus (PVN) of the hypothalamus plays an important role in the control of baroreflex function and sympathetic drive.6 Furthermore, the PVN contains a high density of AT1R7 and is a candidate region to respond to Ang II in the cerebrospinal fluid.8
Several lines of evidence show that reduced nicotinamide-adenine dinucleotide phosphate (NAD[P]H) oxidase-derived reactive oxygen species (ROS) are novel mediators of Ang II signaling in the central nervous system.9 Pretreatment with adenoviral-mediated superoxide dismutase prevents Ang II–induced hypertension and the increased superoxide production.10 On the other hand, the AT1R antagonist losartan abolishes Ang II–stimulated superoxide generation.9 Increases in superoxide production may contribute to impaired arterial baroreflex function.5,11 Conversely, antioxidant treatment improves baroreflex sensitivity.11
Exercise training (EX) has been demonstrated to alter neural control of the circulation, including influencing arterial baroreflex function.12 Although the effects of EX on baroreflex function in normal subjects and animals are increased,12 decreased,13,14 or unchanged,15 EX consistently increases baroreflex sensitivity in CHF and hypertensive subjects.16–18 The mechanisms responsible for the training-induced improvement of baroreflex function involve changes in central and peripheral components of the baroreflex arc. It has been shown that EX not only increases baroreceptor sensitivity16 and decreases ROS in peripheral tissue19,20 but also decrease central sympathetic outflow.17,21 Furthermore, EX improves NO bioactivity within the PVN in CHF,22 which contributes to sympathoinhibition. In addition, EX also reduces AT1R mRNA expression in central cardiovascular regulatory regions, such as the PVN, the nucleus of the solitary tract (NTS), and the rostral ventrolateral medulla (RVLM) in CHF animals.18 However, the central mechanisms mediating baroreflex function after EX are not well understood. In this study, we hypothesized that EX improves arterial baroreflex function via reducing central ROS in a rat model of chronic icv infusion of Ang II. To test this hypothesis, we examined the effects of both EX and central superoxide anion on arterial baroreflex function and measured the central superoxide production and protein expression of AT1R and NAD(P)H oxidase subunits in Ang II–infused rats.
Methods
Animals
Eighty-eight male Sprague–Dawley rats weighing 300 to 310 g were assigned randomly to 4 groups (n = 22 in each group): non-EX Ang II–infused rats, non-EX artificial cerebrospinal fluid (aCSF)–infused rats, EX Ang II–infused rats, and EX aCSF-infused rats. Experiments were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Experimental Animals of the American Physiological Society and the National Institutes of Health.
EX Protocol
Rats were trained on a treadmill 5 days per week for ≈3 to 4 weeks according to the program used by Zheng et al.22 The treadmill speed and incline were gradually increased from 10 m/min, 0% grade, to 25 m/min, 10% grade. Exercise duration was increased from 10 to 20 min/day to 60 min/day to produce a significant endurance effect during EX. To test the efficiency of EX, the soleus muscle was taken at the end of EX and frozen at −80°C. The citrate synthase activity from muscle homogenate was measured spectrophotometrically.23
icv Infusion of Ang II
Three days before the end of EX, the rats were anesthetized with a ketamine/xylazine mixture (90 mg/kg and 5 mg/kg, IP) and placed in a stereotaxic apparatus (Stoelting). After the bregma was identified, a sterile brain cannula using the Alzet Brain Infusion Kit II connected to an osmotic minipump (model 1003D) was inserted into the right lateral cerebral ventricle and fixed to the skull with dental cement. The periosteums of both sides were sutured together to fasten the brain cannula. The coordinates were determined from the Paxinos and Watson rat atlas,24 which were 0.8-mm posterior, 1.4-mm lateral to the bregma and 3.8-mm ventral to the 0 level. The brain cannula, connecting catheter and minipump, were prefilled with Ang II or aCSF. Ang II was delivered at a rate of 60 ng/μL per hour. aCSF and Ang II were infused at 1 μL/h through the minipump. Twenty hours after implantation, the rats continued to be trained. In a preliminary study, the cannula tip placement was confirmed by infusion of 2% Chicago blue dye at the end of the experiment. Water intake was measured daily during infusion of Ang II or aCSF.
Acute Experiments
General Animal Preparation
Twenty-four hours after the end of EX, the rats were anesthetized with urethane (800 mg/kg, IP) and α-chloralose (40 mg/kg, IP). The trachea was intubated, and the rats were artificially ventilated by inhalation of an air–O2 mixture using a ventilator (model SAR-830, Ardmore, PA). The right common carotid artery was catheterized with a pressure transducer (model SPR-524, Millar Instruments) for measurement of mean arterial pressure (MAP). Heart rate (HR) was derived from the arterial pressure pulse using the cardiotachometer function of the PowerLab data acquisition system (model 16SP, AD Instruments). The femoral veins were cannulated bilaterally for administration of drugs. The rat head was stabilized in a stereotaxic apparatus (Stoelting). After the minipump was removed, the brain cannula was connected to a microsyringe (model 7001, Hamilton) and flushed with 2 μL of aCSF.
Recording of Renal Sympathetic Nerve Activity
The renal sympathetic nerve activity (RSNA) was recorded as described by Gao et al.25 The left kidney was exposed retroperitoneally. A branch of the renal nerve was isolated and placed on a bipolar platinum electrode. RSNA was amplified (× 1000) and filtered (bandwidth: 30 to 1000 Hz) via a Grass P55C preamplifier. The nerve signal was further amplified (5 to 10 mV/DIV), filtered (30 to 1000 Hz), and monitored on a Tektronix oscilloscope (model 7313). The signal from the oscilloscope was displayed on a computer where RSNA was rectified, integrated, sampled (1 kHz), and converted to a digital signal by the PowerLab data acquisition system. When an optimal nerve signal was observed, the nerve and electrode were covered with Kwik-Sil gel (World Precision Instruments). The maximum nerve activity was determined by injection of nitroglycerin (60 μg/kg, IV). The noise level for RSNA was determined at the end of the experiment when the rat was dead. The value of RSNA was calculated by subtracting the noise from the recorded value. The baseline RSNA was expressed as a percentage of the maximum.
Assessment of Arterial Baroreflex Function
Baroreflex function was assessed by determining the changes in the RSNA response to decreases and increases in MAP. MAP was initially decreased by intravenous injection of nitroglycerin (25 μg) and then immediately elevated by phenylephrine (10 μg). The injection duration of each drug is 30 to 40 seconds. The data for MAP and RSNA (expressed as a percentage of maximum nerve activity) were collected every 2s over the MAP range from ≈50 to ≈ 140 mm Hg. The resultant sigmoidal relationship of RSNA and MAP was analyzed using a nonlinear regression equation26: %RSNA = A/[1 + exp[B(MAP-C)]] + D where A is the RSNA range (maximum response minus minimum response); B is the slope coefficient (average slope of the reflex); C is the pressure at the midrange of the curve (BP50); and D is the minimum response of RSNA. Values of A to D were derived in each rat, and these values were averaged to give a mean value that was used to construct a composite sigmoidal baroreflex curve. The gain of the function at any give MAP was calculated from the derivative of the above equation. The maximum gain (or peak slope) was calculated using the following equation26: Gainmax = A × B × 1/4, where A is the range and B is the average slope.
Losartan (500 nmol in 1 μL; n = 8 in each group) was injected through the brain cannula. The arterial baroreflex was evaluated before and after losartan. In separate groups, the NAD(P)H oxidase inhibitor apocynin (600 nmol in 1 μL/min; n = 6 in each group) was infused through the brain cannula for 10 minutes. The arterial baroreflex was assessed before and after apocynin. After each icv injection, the brain cannula was flushed with 2 μL of aCSF. At the end of the experiment, the brain was removed and immediately frozen at −80°C for Western blot analysis.
Western Blot Analysis of AT1R and NAD(P)H Oxidase in the PVN
The brains were cut into 100-μm coronal sections with a cryostat (Leica) at −18°C, and 6 consecutive sections at the level of the PVN were collected. The PVN was punched27 and homogenized in radioimmunoprecipitation assay buffer. The protein concentration was measured using a protein assay kit (Pierce Chemical). The proteins were loaded onto a 10% SDS-PAGE gel along with protein standards (Bio-Rad Laboratories) in a separate lane for electrophoresis and then transferred to polyvinylidene fluoride membrane. The membrane was probed with rabbit polyclonal antibody against AT1R, gp91phox, p67phox, p47phox, p22phox (1:500 to 1:1000 dilutions, Santa Cruz Biotechnology) and secondary antibody of goat anti-rabbit IgG (1:5000 dilutions, Pierce Chemical). The protein signals were detected by enhanced chemiluminescence reagent (Pierce Chemical) and analyzed using UVP BioImaging Systems. The levels of target proteins were normalized with GAPDH as a loading control (1:1000 dilutions, Santa Cruz Biotechnology).
Measurement of Superoxide Production in the Hypothalamus and the PVN
In 4 additional groups, the superoxide production in the hypothalamus was measured using lucigenin-enhanced chemiluminescence.28 Rats (n = 6 for each group) were anesthetized and the brain was removed and immersed in cold Krebs/HEPES buffer (pH 7.4)28 on ice. The hypothalamus papilla between the optic chiasm and the pons was dissected and cut into small pieces. One piece of hypothalamus was placed in preheated Krebs/HEPES buffer (37°C) containing 5 μmol/L of lucigenin and then read in a Sirius luminometer, which reports relative light units emitted over a 30-s interval for 5 minutes. The value was subtracted from background activity and normalized to tissue weight. NAD(P)H (10 μmol/L) was used to stimulate NAD(P)H oxidase. To determine the source of superoxide, hypothalamic samples were preincubated with the NAD(P)H oxidase inhibitor apocynin (1mmol/L) and the NO synthase inhibitor NG-monomethyl-L-arginine (1 mmol/L), respectively, for 30 minutes.
To detect superoxide in situ, the brain (n = 2 for each group) was removed and immediately frozen at −80°C for 1 hour, blocked in the coronal plane, and sectioned into 30-μm slices with a cryostat. The sections at the level of the PVN were mounted on microscope slides and incubated with dihydroethidium (2 μmol/L, Molecular Probes) for 30 minutes at 37°C in a light-protected humidified chamber. Images were visualized using a fluorescent microscope (Leica).
Drugs
Apocynin and NG-monomethyl-L-arginine were obtained from the Calbiochem Co. Losartan was a gift from Merck and Co. Ketamine was obtained from Ft Dodge Laboratories Inc. Nitroglycerin was obtained from ETHEX Co. Ang II and other chemicals were obtained from Sigma-Aldrich Co.
Statistical Analyses
Data are presented as mean ± SE. Differences between groups were determined by a 2-way ANOVA followed by the Newman–Keuls test for posthoc analysis of significance. Responses before and after a given intervention were compared with a paired t test. A P < 0.05 was considered statistically significant.
Results
Responses to EX
Table 1 shows that 3 to 4 weeks of EX significantly increased citrate synthase activity in soleus muscle in Ang II- and aCSF-infused rats compared with their respective non-EX groups, indicating an increase in skeletal muscle oxidative capacity and adaptation to EX. Basal MAP, HR, and body weight were not different between EX groups and non-EX groups. The ratios of left ventricular weight to body weight were not altered in EX groups compared with non-EX groups.
TABLE 1.
Baseline Characteristics of EX and Non-EX Rats Infused With Either icv aCSF or Ang II
| Non-EX
|
EX
|
|||
|---|---|---|---|---|
| Parameters | aCSF | Ang II | aCSF | Ang II |
| Body weight, g | 408 ± 9 | 397 ± 5 | 381 ± 6 | 376 ± 6 |
| Left ventricle, g | 0.85 ± 0.03 | 0.77 ± 0.04 | 0.81 ± 0.06 | 0.9 ± 0.05 |
| LV/BW, mg/g | 2.1 ± 0.1 | 1.9 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 |
| Basal MAP, mm Hg | 88.7 ± 5.3 | 95.7 ± 3.8 | 86.1 ± 4.2 | 89.4 ± 5.1 |
| Basal HR, bpm | 375 ± 13 | 392 ± 10 | 360 ± 14 | 370 ± 9 |
| Basal RSNA, % of maximum | 40.6 ± 5.3 | 67.4 ± 4.7* | 37.9 ± 4.3 | 46.2 ± 6.0† |
| Water intake, mL/kg per day | 45.7 ± 3.4 | 61.8 ± 3.5* | 48.5 ± 4.1 | 62.2 ± 3.0* |
| CS, μmol/min per gram | 14.4 ± 3.0 | 15.0 ± 1.5 | 21.3 ± 1.3* | 24.3 ± 2.2* |
Values are mean ± SE. n = 14 in each group. LV indicates left ventricle; BW, body weight; CS, citrate synthase activity.
P < 0.05 vs non-EX aCSF-infused rats;
P < 0.05 vs non-EX Ang II–infused rats.
Effects of EX on RSNA and Water Intake in Ang II–Infused Rats
In non-EX rats, chronic icv infusion of Ang II increased the baseline RSNA and water intake by 66% and 35% compared with aCSF infusion, indicating that changes in non-EX Ang II–infused rats are significantly different from non-EX aCSF-infused rats, as reflected in Table 1. In contrast in EX rats, chronic Ang II infusion did not evoke the increase in baseline RSNA but still increased water intake. There was no difference in the baseline RSNA between EX Ang II–infused rats and EX or non-EX aCSF-infused rats (Table 1).
Effects of EX on Arterial Baroreflex Function in Ang II–Infused Rats
The original recordings of baroreflex control of RSNA in the 4 groups are shown in Figure 1. It is evidence that the reflex RSNA response to phenylephrine in non-EX Ang II–infused rats was attenuated compared with non-EX aCSF-infused rats. There was no difference in the reflex RSNA response between the EX Ang II group and the EX aCSF group. The maximum gain of baroreflex control of RSNA in the non-EX Ang II group was decreased compared with the non-EX aCSF group, as shown in Figure 2. Baroreflex function was restored in the EX Ang II–infused rats compared with the non-EX Ang II–infused rats. Baroreflex gain in the non-EX aCSF group was similar to that of the EX aCSF group.
Figure 1.
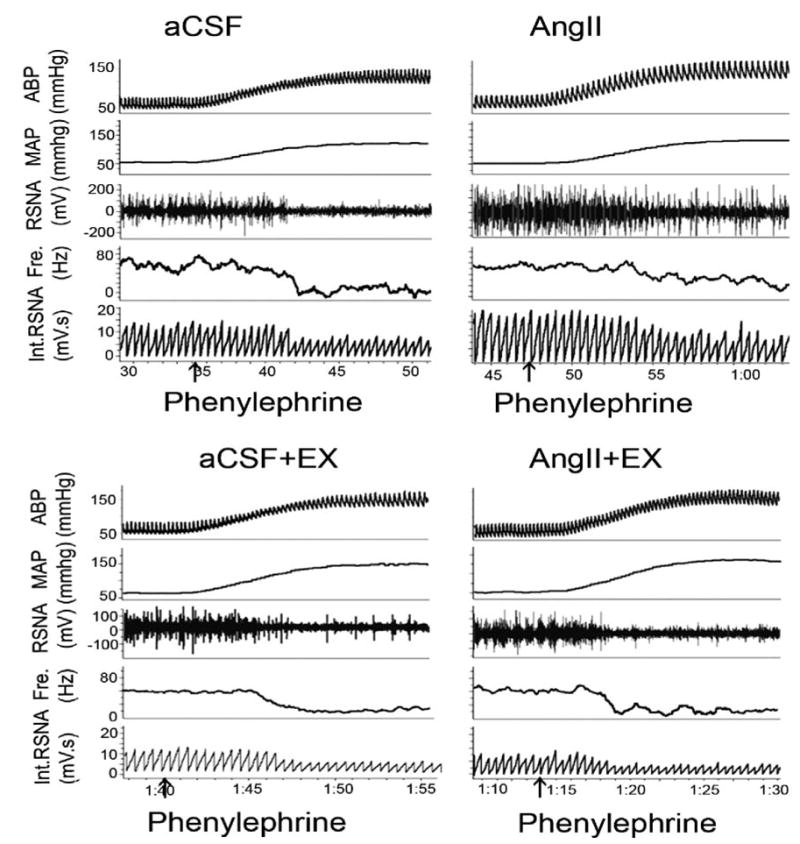
An original recording of the RSNA response to changes in arterial blood pressure induced by nitroglycerin (25 μg IV) and then phenylephrine (10 μg IV). An attenuated RSNA response to an increase in MAP was observed in non-EX Ang II–infused rats (top) compared with non-EX aCSF-infused rats. However, there were no differences of RSNA between EX rats infused with aCSF or Ang II (bottom).
Figure 2.
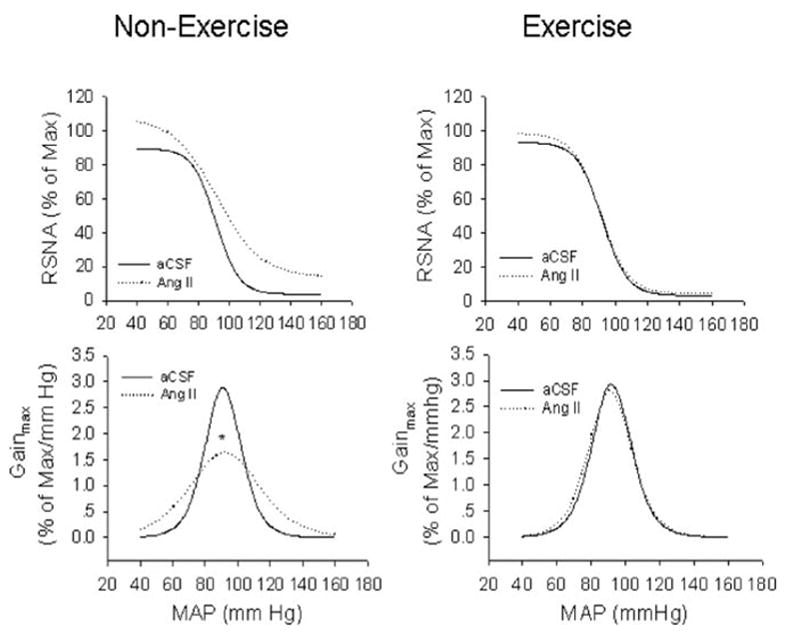
Composite arterial baroreflex curves for control of RSNA in 4 groups of rats. Solid line represents aCSF infusion; dotted line represents Ang II infusion. EX prevented a reduction of baroreflex sensitivity in Ang II–infused rats. Bottom, baroreflex gain curves for each group. *P < 0.05 vs aCSF-infused rats. n = 14 in each group.
Effects of Losartan and Apocynin on Baroreflex Function
Table 2 shows that acute icv infusion of losartan increased the maximum gain and decreased the minimum RSNA only in non-EX Ang II–infused rats. Losartan did not alter baroreflex function in EX Ang II–infused rats, EX aCSF-infused rats, and non-EX aCSF-infused rats. Meanwhile, losartan decreased the basal RSNA in the non-EX Ang II group (31.4 ± 5.8% of maximum versus 67.4 ± 4.7% of maximum; n = 8; P < 0.05) but not in the EX Ang II group (37.1 ± 5.7% of maximum versus 46.2 ± 6.0% of the maximum; P > 0.05).
TABLE 2.
Effects of Losartan (icv) on Arterial Baroreflex Function in Ang II–Infused Rats With or Without EX
| Group | Range, % of Maximum | BP50, mm Hg | Minimum RSNA, % of Maximum | Gainmax, % of Maximum/mm Hg |
|---|---|---|---|---|
| Non-EX Ang II infusion | ||||
| Control | 90.4 ± 4.6 | 88.4 ± 6.1 | 17.3 ± 1.7 | 1.6 ± 0.1 |
| Losartan | 102.7 ± 5.0 | 90.5 ± 6.0 | 8.9 ± 1.2* | 3.3 ± 0.1* |
| EX Ang II infusion | ||||
| Control | 98 ± 5.6 | 89.1 ± 5.4 | 7.1 ± 0.8† | 3.2 ± 0.1† |
| Losartan | 103.4 ± 5.1 | 89.6 ± 3.8 | 6.2 ± 0.6† | 3.4 ± 0.1† |
Values are mean ± SE. n = 8 for each group. BP50 indicates pressure at the midrange of the curve.
P < 0.05 losartan vs control;
P < 0.05 vs control in non-EX rats.
In separate groups, icv infusion of apocynin improved the maximum gain of the baroreflex and minimum RSNA in non-EX Ang II–treated rats (Table 3). There were no differences in baroreflex sensitivity before and after administration of apocynin in non-EX and EX aCSF-infused rats. Apocynin also reduced the basal RSNA in the non-EX Ang II group (33.4 ± 6.4% of maximum versus 62.2 ± 8.9% of maximum; n = 6; P < 0.05) but not in EX Ang II groups (25.7 ± 3.3% of maximum versus 34.9 ± 4.5% of maximum; P > 0.05).
TABLE 3.
Effects of Apocynin (icv) on Arterial Baroreflex Function in Ang II–Infused Rats With or Without EX
| Group | Range, % of Maximum | BP50, mm Hg | Minimum RSNA, % of Maximum | Gainmax, % of Maximum/mm Hg |
|---|---|---|---|---|
| Non-EX Ang II infusion | ||||
| Control | 91.6 ± 4.1 | 89.2 ± 3.6 | 18.5 ± 2.1 | 1.8 ± 0.1 |
| Apocynin | 99 ± 6.2 | 90.5 ± 4.6 | 11.4 ± 1.1* | 3.0 ± 0.1* |
| EX Ang II infusion | ||||
| Control | 100.7 ± 4.3 | 90.7 ± 3.4 | 9.6 ± 1.1† | 3.0 ± 0.1† |
| Apocynin | 103.4 ± 5.1 | 91.5 ± 4.5 | 7.5 ± 1.0† | 3.2 ± 0.1† |
Values are mean ± SE. n = 6 for each group. BP50 indicates pressure at the midrange of the curve.
P < 0.05 apocynin vs control;
P < 0.05 vs control in non-EX rats.
Effects of EX on Superoxide Production in the Hypothalamus and the PVN
The basal superoxide level was lower in EX Ang II–infused rats than in non-EX Ang II–infused rats, which was similar to that in non-EX aCSF-infused rats (Figure 3A). In the presence of NAD(P)H (10 μmol/L), EX also depressed the increase in superoxide production in Ang II–infused rats (Figure 3B). Pretreatment with apocynin markedly reduced superoxide generation in both basal conditions and in the presence of NAD(P)H in non-EX Ang II–infused rats. However, NG-monomethyl-L-arginine (1mmol/L) did not alter superoxide production both in basal conditions and the presence of NAD(P)H in non-EX Ang II–infused rats and non-EX aCSF-infused rats. These data indicate that Ang II–induced superoxide is mainly derived from activation of NAD(P)H oxidase.
Figure 3.
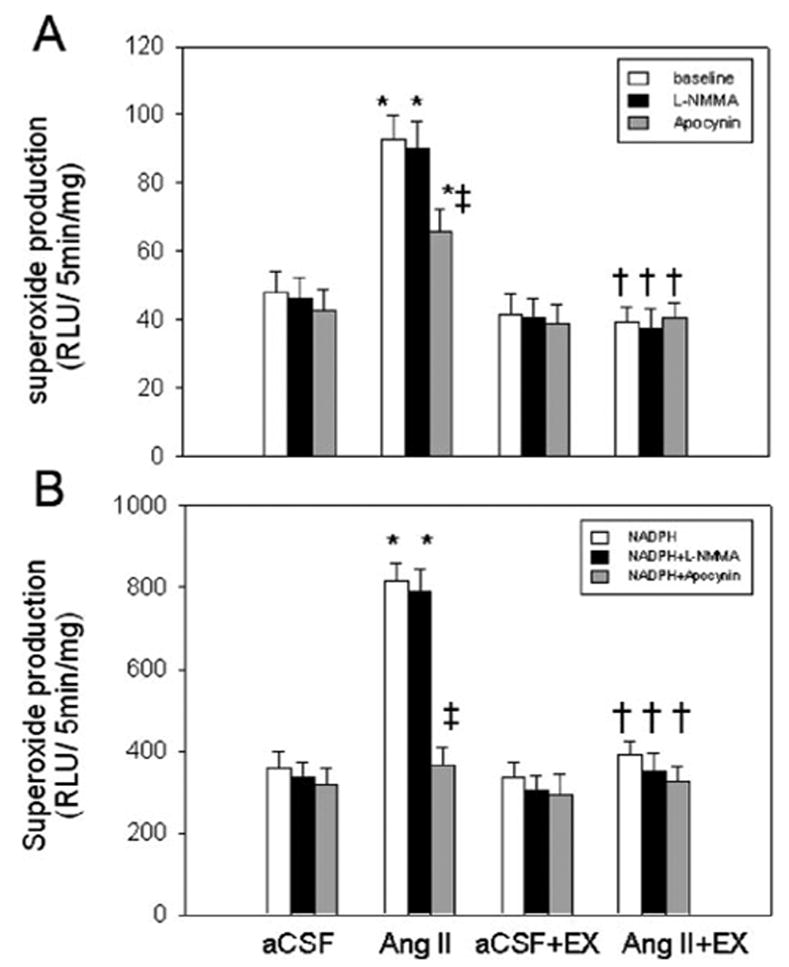
Hypothalamic superoxide production in the basal state (A) or in the presence of NAD(P)H (10 μmol/L; B). Apocynin (1 mmol/L) and NG-monomethyl-L-arginine NG-monomethyl-L-arginine (1 mmol/L) were used to inhibit the activity of NAD(P)H oxidase and NOS, respectively. *P < 0.001 vs aCSF-infused rats; †P < 0.001 vs Ang II–infused rats; ‡P < 0.001, comparison within a group. n = 6, in each group.
In situ detection of superoxide using dihydroethidium fluorescence indicated an intense signal in the PVN. Furthermore, superoxide fluorescence in the PVN was lower in non-EX aCSF-infused rats (Figure 4A) than that in non-EX Ang II–infused rats (Figure 4B). There was no difference of superoxide fluorescence in the PVN between EX aCSF-infused rats (Figure C) and EX Ang II–infused rats (Figure D).
Figure 4.
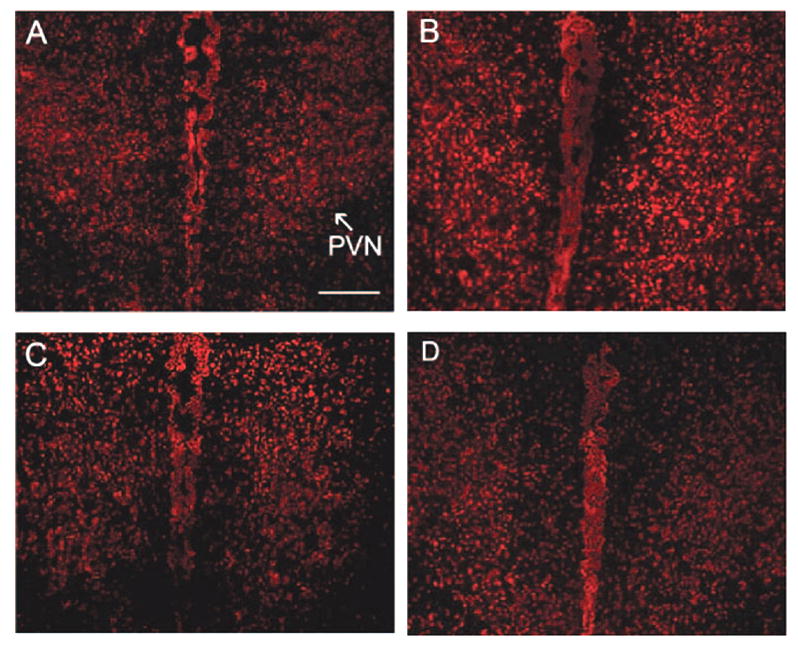
Effect of EX on superoxide production in the PVN in chronic Ang II–infused rats. An intense dihydroethidium fluorescent signal is clearly visible in non-EX Ang II–treated rats compared with all other groups. A, Non-EX aCSF-infused rats; B, Non-EX Ang II–infused rats; C, EX aCSF-infused rats; D, EX Ang II–infused rats. Scale bar = 200 μm.
Effects of EX on Protein Expression of AT1R and NAD(P)H Oxidase Subunits in the PVN
As shown in Figure 5, AT1R protein was increased by 48.9% in non-EX Ang II–infused rats compared with non-EX aCSF-infused rats. In EX Ang II–infused rats, AT1R expression was significantly decreased and was similar to that of EX aCSF-infused rats. Similarly, Figure 6 indicates that protein levels of gp91phox and p47phox were increased by 147% and 62.8% in non-EX Ang II–infused rats compared with non-EX aCSF-infused rats; however, no significant difference in p67phox protein was found in non-EX Ang II–infused rats compared with non-EX aCSF-infused rats. In the EX Ang II–infused group, gp91phox and p47phox protein levels decreased to the level observed in the EX aCSF-infused group.
Figure 5.
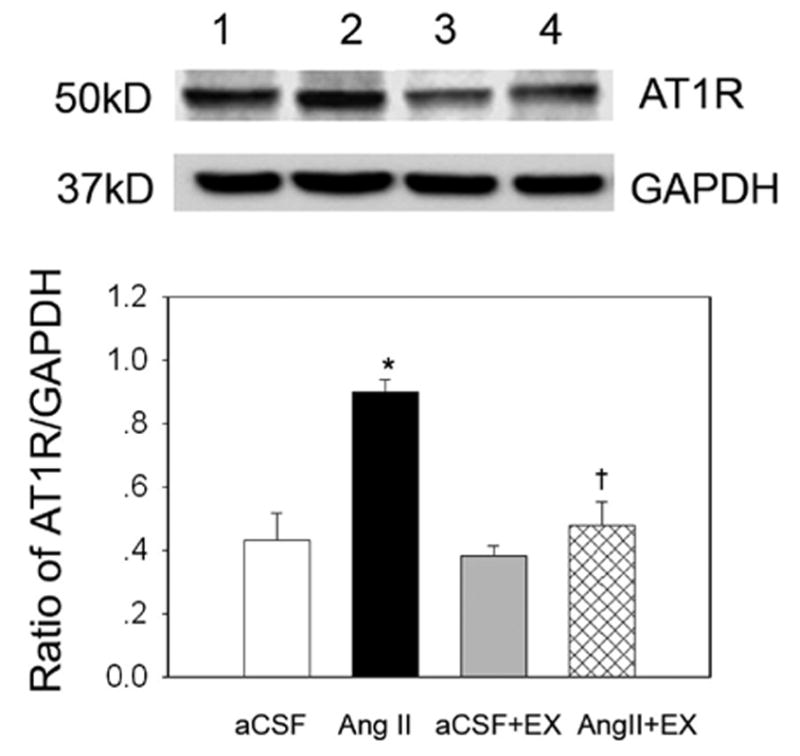
The influence of EX on AT1R protein expression in the PVN in the 4 groups of rats examined. Top, Representative Western blot for AT1R protein (lanes 1 to 4 presents treatment with aCSF, Ang II, aCSF + EX, and Ang II + EX, respectively). Bottom, Mean data from densitometric analysis. Values are mean ± SE. *P < 0.01 vs aCSF; †P < 0.01 vs Ang II. n = 8 in each group.
Figure 6.
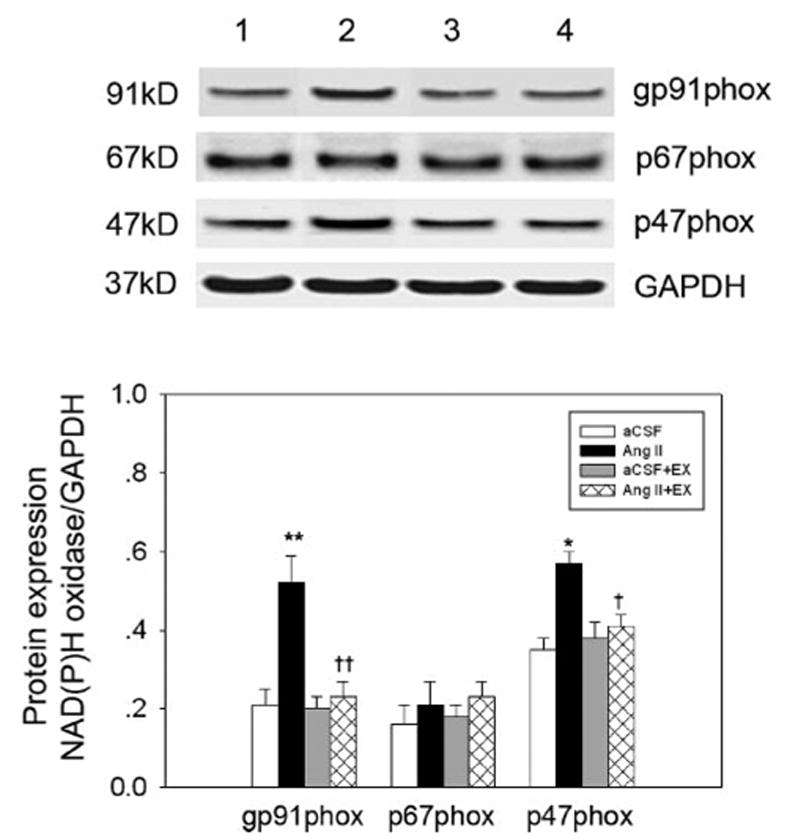
The influence of EX on protein expression of NAD(P)H oxidase subunits in the PVN in the 4 groups of rats studied. Top, Representative Western blot for NAD(P)H subunit expression (lanes 1 to 4 are aCSF, Ang II, aCSF + EX, and Ang II + EX, respectively). Bottom, Mean data from densitometric analysis. Values are mean ± SE. *P < 0.05, **P < 0.01 vs aCSF; †P < 0.05, †† P < 0.01 vs Ang II. n = 6 in each group.
Discussion
Four major findings emerged from this study. First, chronic icv infusion of Ang II significantly decreased arterial baroreflex sensitivity and increased baseline RSNA. These effects were abolished by icv administration of losartan and the NAD(P)H oxidase inhibitor apocynin. Second, chronic icv Ang II infusion increased superoxide production in the PVN concomitant with upregulation of AT1R protein, as well as the NAD(P)H oxidase subunits gp91phox and p47phox. Third, EX prevented Ang II–induced impairment of the arterial baroreflex and increase in RSNA. Finally, EX decreased superoxide phox production, as well as AT1R protein expression and gp91 and p47phox subunits, only in Ang II–infused rats. These results suggest that, in the conditions of high brain Ang II, EX improves baroreflex function by reducing central oxidative stress.
A previous study5 and the results from the current study have confirmed that icv infusion of Ang II depresses arterial baroreflex function. The present study showed that the attenuated baroreflex control of RSNA was primarily because of the decreased gain of the baroreflex and an increase in the minimum RSNA. The maximum RSNA achieved during a decrease in MAP (nitroglycerin administration) was increased so that the range of the baroreflex curve was not changed. Chronic Ang II infusion increased resting RSNA and water intake without a rise in resting MAP. After 3 to 4 weeks of EX, Ang II failed to cause a decrease in the baroreflex gain but increased water intake. Furthermore, the maximum and minimum RSNAs were decreased in the EX Ang II group compared with the non-EX Ang II group. There was no difference in these parameters between EX and non-EX aCSF-infused rats. This reflects the fact that basal levels of RSNA were altered in different groups and started from higher basal RSNA in the non-EX Ang II group and lower basal RSNA in the EX Ang II, EX aCSF, and non-EX aCSF groups. The reduced maximum gain of the baroreflex was restored in EX Ang II–infused rats and reached the level of non-EX aCSF infusion. This is in agreement with previous results obtained in CHF and hypertensive animals after EX.16–18 The effects of EX on baroreflex function in normal subjects has been inconsistent, with either an increase12,16 or decrease13,14 being reported. This discrepancy may be because of differences in the EX workload, the way of testing baroreflex function, and whether the animal is in the anesthetized or conscious state. Regardless of the reported different effects of EX on baroreflex function under normal conditions, EX appears to improve arterial baroreflex function, at least in the conditions of high brain Ang II.
The mechanisms by which EX prevents the impairment of baroreflex function induced by chronic central Ang II infusion are not clear. In view of the fact that resting RSNA was decreased after EX, the reduced central sympathetic drive may play an initial role in the improvement of baroreflex function. The hypothalamic PVN is a key site for control of sympathetic outflow and the regulation of cardiovascular reflexes. PVN neurons project to the RVLM, NTS, and intermediolateral cell column of the spinal cord.29 Michelini30 and Jackson et al31 demonstrated that the oxytocinergic and vasopresinergic projections from the PVN to the NTS were enhanced after EX, which might change neuronal activity in the NTS. A previous study has shown that EX downregulates AT1R mRNA expression in central cardiovascular regulatory regions, such as the PVN, the NTS, and the RVLM, in CHF animals.18 In this study, we focused on the PVN and found that the AT1R protein level in the PVN was significantly lower in the EX Ang II group than in the non-EX Ang II group. However, there was no difference in AT1R protein between the EX and non-EX aCSF groups. Consistent with this result, icv infusion of the AT1R antagonist losartan improved baroreflex function in the non-EX Ang II group but not in the EX Ang II, EX aCSF, and non-EX aCSF groups, which exhibited lower AT1R expression. These data suggest that EX can inhibit sympathoexcitation in the PVN under the conditions of sympathetic overactivity. Because of angiotenergic projections from the PVN to the RVLM,32 we speculate that EX inhibits exogenous Ang II action in the PVN, which, in turn, affects baroreflex integration in the RVLM to alter baroreflex function. Additional studies have shown that EX enhances sympathoinhibition in the PVN via an increase in neuronal NOS expression in CHF rats22 and an increase in the number of NOS-positive neurons in hypertensive rats.33 The roles of PVN-NTS and PVN-RVLM neuronal pathways after EX in the modulation of baroreflex control remain to be examined.
In addition to a central mechanism, EX exerts peripheral effects on the baroreflex. Brum et al16 and Krieger et al12 demonstrated that EX increased baroreceptor afferent activity in normal and spontaneous hypertensive rats. In CHF animals, plasma Ang II was decreased after EX, which positively correlated with sympathetic nerve activity and conversely correlated with arterial baroreflex function.17,18 There is evidence that EX reduces myocardial oxidative stress, contributing to the improved baroreflex control of HR.20 In addition, we cannot exclude the possibility that EX changes the reactivity of effectors, contributing to the improvement of baroreflex function.
A growing body of evidence indicates that superoxide is a novel signaling molecule that mediates central Ang II–induced hypertension, thirst, and sympathoexcitation.9 However, the influence of superoxide in the brain on the arterial baroreflex and the effect of EX on superoxide generation are not identified. In the present study, icv infusion of the NAD(P)H oxidase inhibitor apocynin increased the gain of baroreflex control of RSNA and restored the blunted arterial baroreflex in non-EX Ang II–infused rats where superoxide production in the hypothalamus and the PVN was increased. Importantly, superoxide production in the hypothalamus and the PVN were lower in the EX Ang II group than in the non-EX Ang II group and near to the level of the non-EX aCSF control group. These results suggest that EX-induced baroreflex improvement is associated with a reduction in oxidative stress within the brain.
Superoxide anions are generated by multiple enzymes, such as NAD(P)H oxidase, xanthine oxidase, and uncoupled NOS. Zimmerman et al34 have identified NAD(P)H oxidase as the major source of a central Ang II–induced superoxide increase. In this study, we found that when superoxide level was in the reference range, apocynin did not affect superoxide generation; however, in non-EX Ang II–infused rats, increased superoxide production in the hypothalamus under basal conditions and in the presence of NAD(P)H was suppressed by apocynin.
NAD(P)H oxidase consists of membrane-bound subunits (gp91phox and p22phox) and cytosolic subunits (p40phox, p47phox, p67phox, and rac1). After stimulation of AT1R by Ang II, p47phox is phosphorylated, which mediates assembly of enzyme subunits into the active enzyme complex.35 Although the activity of NAD(P)H oxidase was not determined in the current study, we found that EX prevented the increased protein levels of the NAD(P)H oxidase subunits gp91phox and p47phox in the PVN of Ang II infused rats.
Citrate synthase activity of the soleus muscle was used to assess the effectiveness of EX, which was increased by 47.9% and 62% in EX aCSF- and Ang II–infused rats compared with their respective non-EX groups. These results are similar to a previous report,22 suggesting increased oxidative capacity of skeletal muscle and adaptation to EX. On the other hand, the resting HR has been reported to be reduced16,17,20 or unchanged22,36 after various EX regimens. These differences may be related to different training programs (intensity, duration, and workload), species, and the states of the animal (eg, conscious or anesthetized and normal or diseased). We failed to observe a decrease in resting HR, probably related to anesthesia.
In conclusion, the results of the present study indicate that central Ang II–mediated ROS production is involved in modulation of arterial baroreflex function and that the improvement of baroreflex function after EX is associated with reduced ROS only in conditions of high brain Ang II. These data suggest a preventative role of EX in the pathogenesis of cardiovascular diseases dependent on an increase in central Ang II.
Perspectives
Activation of the renin–angiotensin system plays an important role in various cardiovascular diseases, including hypertension, CHF, diabetes mellitus, and coronary artery disease. Although pharmacological therapies have had some success in reducing the mortality of these diseases, the morbidity rate from these diseases remains high. EX may be an important factor in the prevention and treatment of cardiovascular diseases. In the present study, we demonstrated that EX prevents the impairment of the arterial baroreflex induced by chronic Ang II infusion, which is associated with a reduction in oxidative stress in the brain. The current study indicates that 1 of the mechanisms for the improvement of cardiovascular function by EX involves the Ang II–NAD(P)H–ROS pathway. This finding also supports EX as adjunctive therapy in several cardiovascular diseases where Ang II is known to be increased.
Footnotes
Disclosures
None.
Source of Funding
This study was supported by National Heart, Lung, and Blood Institute Grant PO1-HL-62222.
References
- 1.Grassi G, Seravalle G, Dell’Oro R, Facchini A, Ilardo V, Mancia G. Sympathetic and baroreflex function in hypertensive or heart failure patients with ventricular arrhythmias. J Hypertens. 2004;22:1747–1753. doi: 10.1097/00004872-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Chen JS, Zucker IH. Carotid sinus baroreceptor sensitivity in experimental heart failure. Circulation. 1990;81:1959–1966. doi: 10.1161/01.cir.81.6.1959. [DOI] [PubMed] [Google Scholar]
- 3.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 4.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol. 1995;269:R1189–R1196. doi: 10.1152/ajpregu.1995.269.5.R1189. [DOI] [PubMed] [Google Scholar]
- 5.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 6.Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst. 1988;22:211–219. doi: 10.1016/0165-1838(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 7.Allen AM, Moeller I, Jenkins TA, Zhuo J, Aldred GP, Chai SY, Mendelsohn FA. Angiotensin receptors in the nervous system. Brain Res Bull. 1998;47:17–28. doi: 10.1016/s0361-9230(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 8.Porter JP. Chronic intracerebroventricular infusion of angiotensin II increases brain AT1 receptor expression in young rats. Brain Res Dev Brain Res. 1999;112:293–295. doi: 10.1016/s0165-3806(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Mao HZ, Abboud FM, Chapleau MW. Oxygen-derived free radicals contribute to baroreceptor dysfunction in atherosclerotic rabbits. Circ Res. 1996;79:802–811. doi: 10.1161/01.res.79.4.802. [DOI] [PubMed] [Google Scholar]
- 12.Krieger EM, Da Silva GJ, Negrao CE. Effects of exercise training on baroreflex control of the cardiovascular system. Ann N Y Acad Sci. 2001;940:338–347. doi: 10.1111/j.1749-6632.2001.tb03689.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen CY, DiCarlo SE, Scislo TJ. Daily spontaneous running attenuated the central gain of the arterial baroreflex. Am J Physiol. 1995;268:H662–H669. doi: 10.1152/ajpheart.1995.268.2.H662. [DOI] [PubMed] [Google Scholar]
- 14.DiCarlo SE, Bishop VS. Exercise training attenuates baroreflex regulation of nerve activity in rabbits. Am J Physiol. 1988;255:H974–H979. doi: 10.1152/ajpheart.1988.255.4.H974. [DOI] [PubMed] [Google Scholar]
- 15.Sheldahl LM, Ebert TJ, Cox B, Tristani FE. Effect of aerobic training on baroreflex regulation of cardiac and sympathetic function. J Appl Physiol. 1994;76:158–165. doi: 10.1152/jappl.1994.76.1.158. [DOI] [PubMed] [Google Scholar]
- 16.Brum PC, Da Silva GJ, Moreira ED, Ida F, Negrao CE, Krieger EM. Exercise training increases baroreceptor gain sensitivity in normal and hypertensive rats. Hypertension. 2000;36:1018–1022. doi: 10.1161/01.hyp.36.6.1018. [DOI] [PubMed] [Google Scholar]
- 17.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 18.Zucker IH, Patel KP, Schultz HD, Li YF, Wang W, Pliquett RU. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci Rev. 2004;32:107–111. doi: 10.1097/00003677-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 20.Irigoyen MC, Paulini J, Flores LJ, Flues K, Bertagnolli M, Moreira ED, Consolim-Colombo F, Bello-Klein A, Angelis KD. Exercise training improves baroreflex sensitivity associated with oxidative stress reduction in ovariectomized rats. Hypertension. 2005;46:998–1003. doi: 10.1161/01.HYP.0000176238.90688.6b. [DOI] [PubMed] [Google Scholar]
- 21.Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann N Y Acad Sci. 2001;940:431–443. [PubMed] [Google Scholar]
- 22.Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H2332–H2341. doi: 10.1152/ajpheart.00473.2004. [DOI] [PubMed] [Google Scholar]
- 23.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- 24.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando, FL: Academic; 1986. [Google Scholar]
- 25.Gao L, Zhu Z, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation impairs baroreflex control of renal sympathetic nerve activity in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1706–H1711. doi: 10.1152/ajpheart.01097.2003. [DOI] [PubMed] [Google Scholar]
- 26.Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- 27.Palkovits M, Brownstein M. Brain microdissection techniques. In: Cuello AC, editor. Brain Microdissection Technique. Chichester, United Kingdom: Wiley; 1983. [Google Scholar]
- 28.Sun H, Molacek E, Zheng H, Fang Q, Patel KP, Mayhan WG. Alcohol-induced impairment of neuronal nitric oxide synthase (nNOS)-dependent dilation of cerebral arterioles: role of NAD(P)H oxidase. J Mol Cell Cardiol. 2006;40:321–328. doi: 10.1016/j.yjmcc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 30.Michelini LC. Oxytocin in the NTS. A new modulator of cardiovascular control during exercise. Ann N Y Acad Sci. 2001;940:206–220. [PubMed] [Google Scholar]
- 31.Jackson K, Silva HM, Zhang W, Michelini LC, Stern JE. Exercise training differentially affects intrinsic excitability of autonomic and neuroendocrine neurons in the hypothalamic paraventricular nucleus. J Neurophysiol. 2005;94:3211–3220. doi: 10.1152/jn.00277.2005. [DOI] [PubMed] [Google Scholar]
- 32.Tagawa T, Dampney RA. AT(1) receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- 33.DiCarlo SE, Zheng H, Collins HL, Rodenbaugh DW, Patel KP. Daily exercise normalizes the number of diaphorase (NOS) positive neurons in the hypothalamus of hypertensive rats. Brain Res. 2002;955:153–160. doi: 10.1016/s0006-8993(02)03400-5. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 35.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- 36.Negrao CE, Irigoyen MC, Moreira ED, Brum PC, Freire PM, Krieger EM. Effect of exercise training on RSNA, baroreflex control, and blood pressure responsiveness. Am J Physiol. 1993;265:R365–R370. doi: 10.1152/ajpregu.1993.265.2.R365. [DOI] [PubMed] [Google Scholar]


