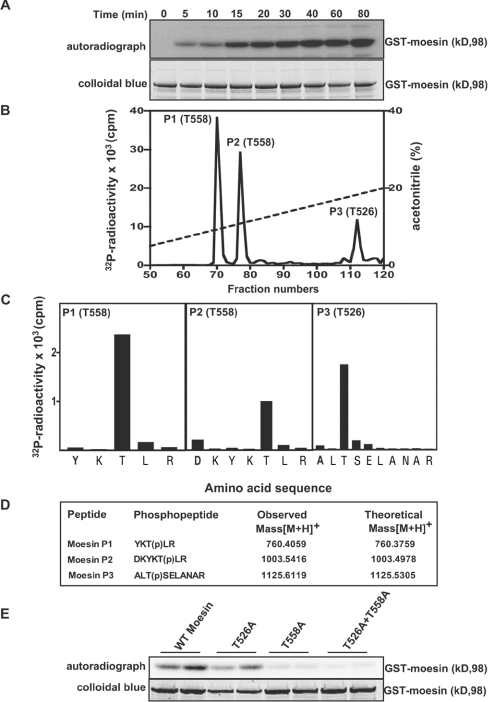
Figure 4. Identification of residues on moesin that are phosphorylated by LRRK2.
(A) E. coli-expressed moesin was incubated at 65°C for 15 min before phosphorylation with GST–LRRK2[1326–2527,G2019S] and [γ-32P]ATP for the indicated times. Phosphorylation of the moesin protein was determined after PAGE and subsequent autoradiography of the Colloidal Blue-stained bands corresponding to moesin. Similar results were obtained in three separate experiments. (B) 32P-labelled moesin after phosphorylation with the GST–LRRK2[1326–2527,G2019S] for 40 min was digested with trypsin and chromatographed on a C18 column. Fractions containing the major 32P-labelled tryptic peptide (P1), peptide P2 and peptide P3 are shown, and no other major 32P-labelled peptides were observed in other fractions of the chromatography. (C) The indicated peptides were subjected to solid-phase sequencing, and the 32P radioactivity released after each cycle of Edman degradation was measured. (D) Peptides were also analysed by MALDI–TOF and MALDI–TOF–TOF-MS (the latter spectra for peptide P2 are shown in Supplementary Figure 1 at http://www.BiochemJ.org/bj/405/bj4050307add.htm) and the inferred amino acid sequence and the site of phosphorylation denoted by ‘(p)’ is indicated, together with the observed and theoretical mass of each peptide. (E) As in (A), except the indicated wild-type and mutant forms of moesin were phosphorylated with GST–LRRK2[1326–2527,G2019S] for 30 min. Similar results were obtained in two separate experiments.