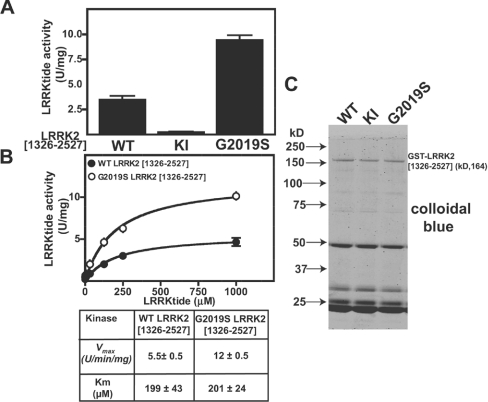
Figure 6. Generation of a peptide substrate for LRRK2.
(A) HEK-293 cells were transfected with constructs encoding the indicated forms of active and kinase-inactive (KI, D2017A) GST–LRRK2. At 36 h after transfection, LRRK2 kinases were affinity-purified, subjected to PAGE and stained with Colloidal Blue to quantify relative protein levels. GST–LRRK2 (1 μg of total protein from each preparation) was assayed by measuring phosphorylation of the LRRKtide peptide (RLGRDKYKTLRQIRQ) at 300 μM as described in the Materials and methods section. Results of the kinase catalytic assays are presented as the mean catalytic activity±S.D. for assays carried out in triplicate. The results presented are representative of two or three independent experiments. (B) As in (A), except that concentrations of LRRKtide were varied in order to enable calculation of the enzymatic parameters Vmax and Km. (C) A 2 μg portion of the indicated forms of GST–LRRK2 assayed in (A) was subjected to PAGE and stained with Colloidal Blue. kD = kDa; U, unit.