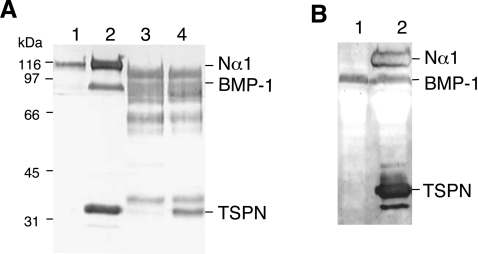
Figure 4. BMP-1 cleavage of the N-propeptide domain of the proα1(V) chain.
(A) Western-blot analysis of BMP-1 processing of the N-propeptide of the proα1(V) chain in HEK-293 cells (lanes 1 and 2) compared with in vitro experiments with purified proteins (lanes 3 and 4). Electrophoretic patterns of HEK-293 cell media transfected with Nα1 construct alone (lane 1) and co-transfected with BMP-1 and Nα1 constructs (lane 2). Electrophoretic patterns of purified recombinant Nα1 fragment incubated without (lane 3) or with (lane 4) recombinant BMP-1. (B) Western-blot analysis of BMP-1 processing of the N-propeptide of the proα1(V) chain in HEK-293 cells expressing BMP-1. Electrophoretic patterns of HEK-293 BMP-1 cell medium (lane 1) and of medium from HEK-293 BMP-1 cells transfected with Nα1 construct (lane 2). Membranes were probed with pAbTSPN and reprobed with anti-BMP-1 polyclonal antibodies. Left: running positions of protein standards are indicated in kDa.