Fig. 2.
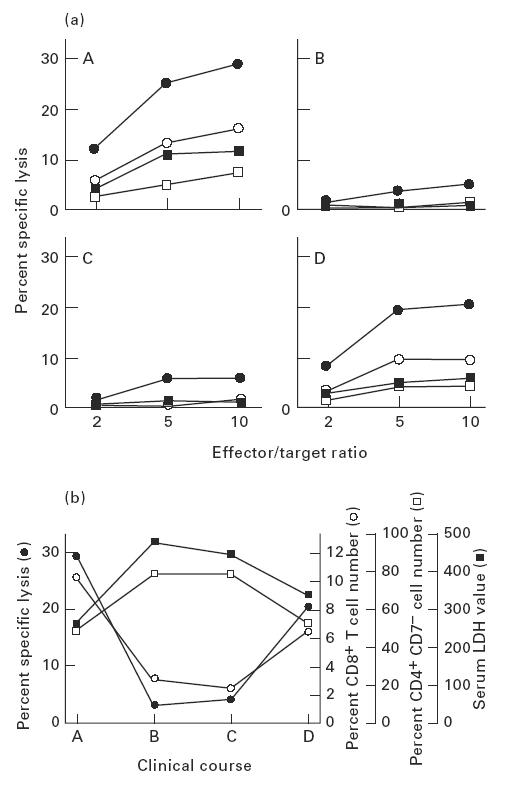
Relationships of cytotoxic activity of cultured CD8+ cells, circulating CD8+ cell percentage, and serum lactate dehydrogenase (LDH) level in the patient's clinical course. (a) CD8+ cells were obtained from the patient at four different time points (A–D) at intervals of 10–14 days during a 2-month clinical period. CD8+ cells separated at each time point were cultured in the presence of rIL-2 (50 U/ml) (□), or rIL-2 (50 U/ml) combined with rIFN-γ (500 U/ml (▪), 1000 U/ml (○), or 5000 U/ml (•)) with mitomycin-C-treated purified Sézary cells. Cytotoxicity of the cultured CD8+ cells (effector cells) was assayed by 51Cr-release of labelled Sézary tumour cells (target cells) at the indicated effector/target ratios. (b) CD8+ cells separated at each time point were cultured in medium supplemented with rIL-2 (50 U/ml) and rIFN-γ (5000 U/ml). Sézary cell-specific cytotoxicity was measured by cytolytic assay at an effector/target ratio of 10 (•). PBMC were stained by fluorescein-conjugated anti-CD8 MoAb and subjected to flow cytometric analysis (○). PBMC were double-stained by fluorescein-conjugated anti-CD7 MoAb and PE-conjugated anti-CD4 MoAb, and percentage of CD4+ CD7− cells was calculated by flow cytometry (□). LDH levels in sera were measured at each time point (▪).
