Abstract
Muscle is an attractive target for gene therapy and for immunization with DNA vaccines and is also the target of immunological injury in myositis. It is important therefore to understand the immunologic capabilities of muscle cells themselves. In this study, we show that proinflammatory stimuli induce the expression of other cytokines such as IL-6, transforming growth factor-beta (TGF-β), and granulocyte-macrophage colony-stimulating factor (GM-CSF) by muscle cells themselves, as well as the up-regulation of human leucocyte antigen (HLA) class I, class II and intercellular adhesion molecule-1 (ICAM-1). Thus, muscle cells have an inherent ability to express and respond to a variety of cytokines and chemokines. The levels of HLA class I, class II and ICAM-1 in inflamed muscle may be affected by the secreted products of the stimulation.
Keywords: human skeletal muscle cells, proinflammatory stimuli, cytokines, chemokines
INTRODUCTION
Although it is conventional to discuss the effects of the immune system on other cells, tissues, or organs, the system's boundaries cannot be sharply drawn, and in an increasing number of ways the immunologic capabilities of non-immune tissues are recognized as determining the course of inflammation. Beyond the need for MHC molecules on cell surfaces to mark a target for lymphocytes, and the presence of professional antigen-presenting cells (APC) in many tissues, highly differentiated non-immune cells make cytokine receptors, adhesion molecules and co-stimulatory molecules, and some tissues use immunologic mechanisms to protect themselves from immunologic injury [1].
Because muscle is an attractive target for gene therapy—myoblasts offer advantages for gene delivery [2] and intramuscular immunization with DNA vaccines [3]—it is important to understand its immunologic capabilities. Muscle has proved resistant to the development of inflammation caused by immunization with heterologous muscle [4], and inflammation does not persist long after acute viral injury [5]. Although knockout of transforming growth factor-beta (TGF-β) causes myositis in mice, the manifestation of deranged immunity is not limited to muscle [6]. Thus, there is no satisfactory animal model for the human idiopathic inflammatory myopathies—polymyositis, dermatomyositis and related diseases.
In an effort to increase the understanding of inflammation in myositis and to help lay the groundwork for the use of muscle as a site for gene therapy and vaccination, we explored the response of muscle cells to immunological stimuli in vitro. In particular, we have measured the synthesis of various cytokines and HLA class I, class II and intercellular adhesion molecule-1 (ICAM-1) expression by human myoblasts and myotubes both constitutively and in response to inflammatory stimuli.
MATERIALS AND METHODS
Antibodies
Mouse monoclonal FITC-conjugated anti-HLA (A, B, C) class I (W6/32), PE-conjugated anti-HLA DP + DQ + DR (IQU9) (Serotech, Raleigh, NC), PE-conjugated anti-human ICAM-1 (CD54) (MEM-111) (Caltag Labs, Burlingame, CA), anti-human N-CAM (CD56) (MY31), and mouse immunoglobulin fluorescence controls (Becton Dickinson, San Jose, CA) were used for flow cytometric analysis.
Muscle cell culture
Normal human skeletal muscle cells from a single donor (Clonetics Corp., San Diego, CA) were maintained in F10 growth medium (Ham's F10 nutrient mixture (Gibco BRL, Gaithersburg, MD) supplemented with 20% fetal calf serum (FCS), 2% chick embryo extract, 100 U/ml penicillin, 100 μg/ml streptomycin). The purity of myoblasts was determined by analysing for the presence of N-CAM (CD56) by flow cytometry (> 96% cells expressed CD56). The cells were maintained at a very low density (0.6–1.0 × 104/cm2) to prevent differentiation due to contact. Myotubes were obtained by culturing myoblasts initially in growth medium and later changing to fusion medium (Dulbecco's minimum essential medium (DMEM) supplemented with 2% horse serum, 100 U/ml penicillin, 100 μg/ml streptomycin) until cells were confluent. Myotubes were maintained in fusion medium for at least 14 days before the addition of cytokines.
Cytokine and chemokine incubations
Myoblasts and myotubes were incubated with cytokines (IL-1α, IL-1β, tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ)) to induce cytokine synthesis. Alternatively, myoblasts and myotubes were incubated for 48 h with cytokines (IL-1α (1 ng/ml), IL-1β (1 ng/ml), TNF-α (1 ng/ml), IFN-γ (500 U/ml), IL-6 (10 ng/ml), TGF-β (10 ng/ml)) or chemokines (MIP-1α (100 ng/ml), MIP-1β (100 ng/ml), RANTES (100 ng/ml), GRO-α (100 ng/ml), IL-8 (100 ng/ml), MCP-1 (100 ng/ml)) to induce HLA class I, HLA class II, and ICAM-1 expression. The effective concentrations of cytokines and chemokines were determined in preliminary experiments (not shown).
Northern blotting
Total RNA was isolated using RNeasy total RNA Kit (Qiagen Inc, Chatsworth, CA). RNA (10 μg) was subjected to 1% denaturing agarose gel electrophoresis followed by blotting onto a nytran membrane (Schleicher & Schuell, Keene, NH). Blots were hybridized with 32P-labelled HLA class I cDNA probe (391 bp). The probe was generated by polymerase chain reaction (PCR) amplification of a muscle cDNA library with primers in exon 4 (sense 5′gaattttctgactcttcccgtcaga3′) and exon 5 (anti-sense 5′acggcagcgaccacagctccagt3′) of the heavy chain of class I (HLA A11). Hybridization was performed overnight at 42°C in Hybrisol 1 solution containing 50% formamide, 10% dextran sulfate, 1% SDS and blocking reagents (Oncor, Gaithersburg, MD). The filter was washed twice at room temperature with 2×SSC/0.5% SDS under gentle shaking for 30 min and once at 55°C for 20 min with 0.2×SSC/0.5% SDS. Finally, the filter was briefly washed with 2×SSC and exposed overnight using Biomax MR single emulsion film (Kodak, Rochester, NY). 32P-labelled human G3PDH cDNA probe (1.1 kb) (Clontech, CA) was used to determine RNA loading.
Reverse transcriptase-PCR
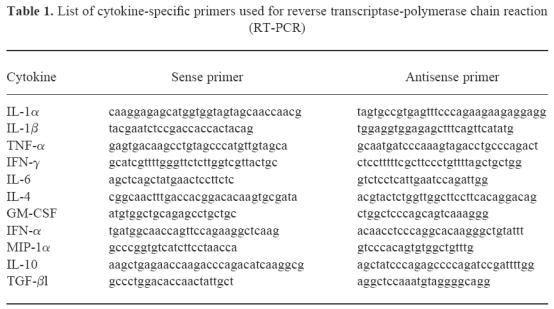
First strand cDNA synthesis for reverse transcriptase (RT)-PCR analysis for cytokines was primed from 1 μg total RNA with 50 ng random hexamers and 20 U AMV reverse transcriptase under conditions suggested by the manufacturer (Boehringer-Mannheim, Indianapolis, IN). PCR was performed with cytokine-specific primers (Clontech) according to the manufacturer's instructions. PCR conditions were as follows: 30–35 cycles at 94°C for 45 s, 60°C for 45 s, and 72°C for 2 min followed by a final extension at 72°C for 7 min. The sequences of the primers are indicated in Table 1. The primers for IL-1β and MIP-1α were a gift from S. Kotake and E. Adams (NIAMS, NIH, Bethesda, MD). Aliquots (10%) of PCR products were analysed on 1.8% agarose gels, and the bands were visualized by ethidium bromide staining.
Table 1.
List of cytokine-specific primers used for reverse transcriptase-polymerase chain reaction (RT-PCR)
ELISAs
Immunoassays were carried out only for the cytokines whose expression was detected by RT-PCR. Assays for TGF-β, MIP-1α, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1β, TNF-α and IFN-α were carried out as per the manufacturers' protocol (Quantikine human TGF-β, MIP-1α, IL-6, GM-CSF, IL-1β (R&D Systems, Minneapolis, MN), TNF-α and IFN-α (Endogen, Woburn, MA). If no cytokine was detected in a culture, the assay was repeated after the supernatant was concentrated 10-fold by speed vac. Each experiment was done twice in triplicate, and the results were expressed as mean ± s.e.m.
Flow cytometry
Cells were harvested using 0.05% trypsin–0.53 mm EDTA in Hanks' balanced salt solution (HBSS), washed twice with PBS containing 2% FCS, 1% bovine serum albumin (BSA) and 0.05% sodium azide (PBS–FBS), and divided into aliquots (5 × 105 cells/ml). The cell pellets were centrifuged for 10 min at 200 g at 4°C, resuspended in 100 μl PBS–FBS, and incubated for 30 min at 4°C on ice with MoAbs against HLA (ABC) class I–FITC, class II DP + DQ + DR–RPE, ICAM–PE, NCAM–PE. The cells were washed three times, resuspended in 1% paraformaldehyde in PBS, and analysed using FACScan (Becton Dickinson). The results were expressed as average fluorescence intensity of the entire population of cells. Three independent experiments were carried out for each of the cytokines or chemokines tested.
RESULTS
Cytokines produced by myoblasts under proinflammatory stimuli
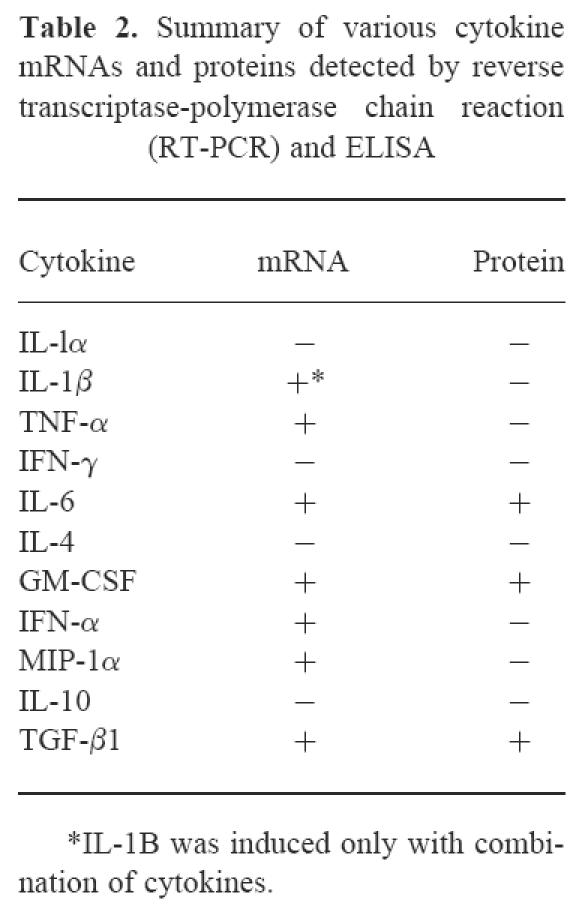
The constitutive low level production of IL-6 (50 ± 4 pg/ml) was increased in a dose-dependent manner under the stimulation of IL-1α, IL-1β, TNF-α and IFN-γ. TNF-α and IL-1α induced higher levels of IL-6 production than did IL-1β and IFN-γ (Fig. 1a). The constitutive low level production of TGF-β (77 ± 4 pg/ml) was increased more by IFN-γ and TNF-α than by IL-1α (Fig. 1b). IL-1β suppressed the basal level of TGF-β synthesis. Myoblasts do not produce GM-CSF constitutively. IL-1α, IL-1β and TNF-α, but not IFN-γ, induced GM-CSF production in a dose-dependent manner. TNF-α and IL-1α induced more than did IL-1β (Fig. 1c).
Fig. 1.
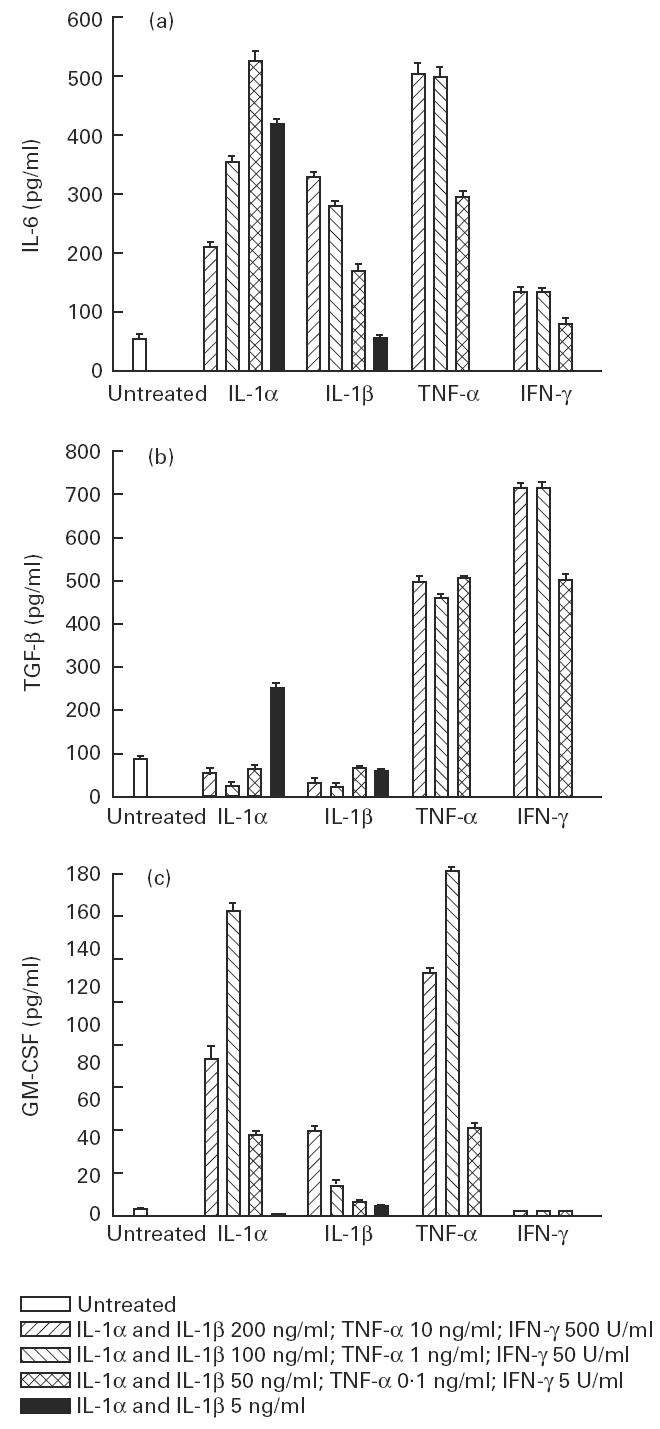
Dose effect of IL-1α, IL-1β, tumour necrosis factor-alpha (TNF-α) and IFN-γ on the production of IL-6 (a), transforming growth factor-beta (TGF-β) (b) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (c) by myoblasts. The values are presented as mean (pg/ml) ± s.e.m. of three determinations in a representative experiment.
Peak levels of IL-6 production were observed at 9 h under TNF-α and IFN-γ stimulation, and between 24 h and 48 h under IL-1α and IL-1β stimulation (Fig. 2a). By contrast, peak levels of TGF-β production were observed at 48 h under TNF-α and IL-1α stimulation and at 9 h under IFN-γ stimulation. IL-1β stimulation did not result in additional synthesis of TGF-β over 48 h (Fig. 2b). Peak levels of GM-CSF production were observed at 24 h under TNF-α, IL-1α and IL-1β stimulation.
Fig. 2.
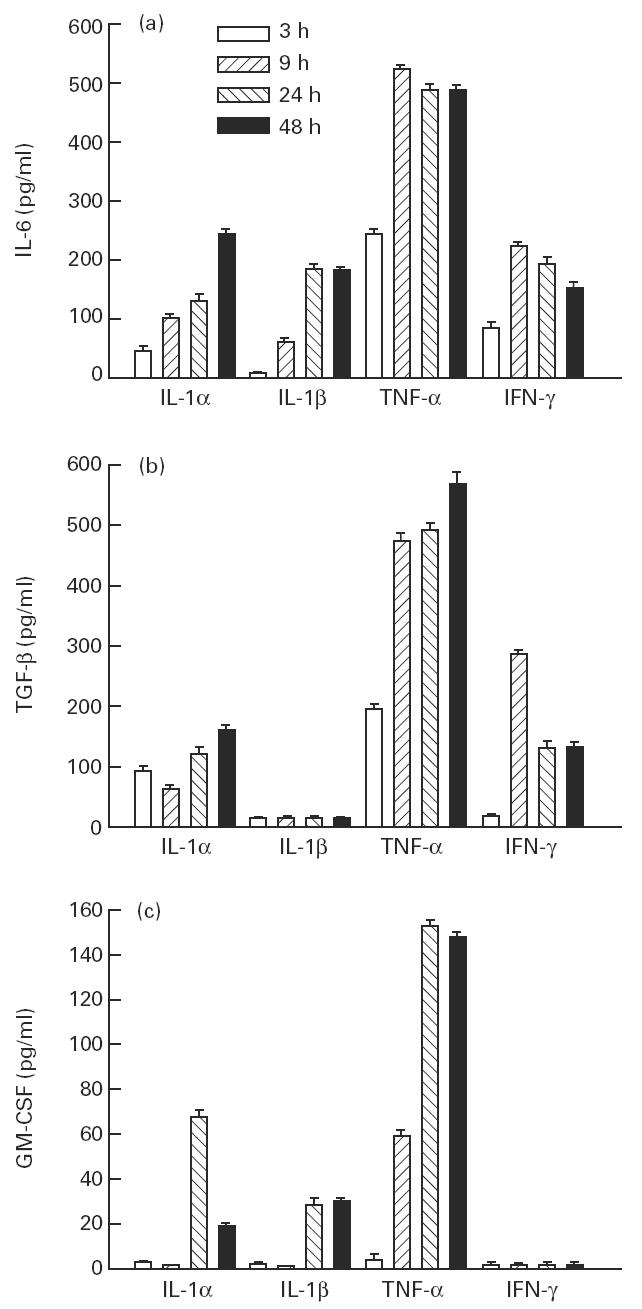
Effect of IL-1-α (50 ng/ml), IL-1β (100 ng/ml), tumour necrosis factor-alpha (TNF-α) (1 ng/ml) and IFN-γ (500 U/ml) on the production of IL-6 (a), transforming growth factor-beta (TGF-β) (b) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (c) by myoblasts. The values are presented as mean (pg/ml) ± s.e.m. of three determinations in a representative experiment.
The quantity of cytokines secreted by muscle cells under proinflammatory conditions varied widely. For IL-6 the level ranged from 7 to 500 pg/ml, for TGF-β it ranged from 21 to 700 pg/ml, and for GM-CSF from 1 to 175 pg/ml. The levels of cytokines secreted by muscle cells were within the biologically effective range [7–9].
Synthesis of cytokine mRNA without cytokine secretion
Under untreated culture conditions in a medium containing 20% FCS and chick embryo extract, myoblasts made detectable mRNAs for MIP-1α and IFN-α (Fig. 3). Individual proinflammatory cytokine stimulation of myoblasts by IL-1α (1 ng/ml), IL-1β (l ng/ml), TNF-α (1 ng/ml), IFN-γ (500 U/ml) or IL-6 (1 ng/ml) induced mRNA expression of TNF-α (Fig. 3) but not of IL-1α, IL-1β, IL-4, IL-10, IL-12, and IFN-γ (data not shown). Similar results were observed for myotubes, except that TNF-α mRNA was not induced by any proinflammatory stimuli (data not shown). IL-1β mRNA expression in myoblasts was induced only by a combination of the proinflammatory stimuli (data not shown). IFN-α, TNF-α, MIP-1α and IL-1β cytokines were not detected in the medium by ELISA even after 10-fold concentration of culture supernatants (the detectable levels for these cytokines are < 3 pg/ml, < 5 pg/ml, < 6 pg/ml, and < 1 pg/ml, respectively). A summary of mRNAs and proteins detected by RT-PCR and ELISA is shown in Table 2.
Fig. 3.
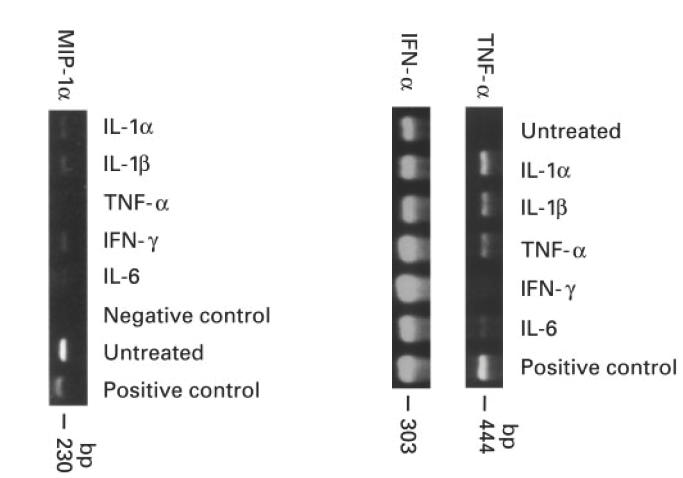
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of the untreated and cytokine-treated muscle cultures for MIP-1α, tumour necrosis factor-alpha (TNF-α) and IFN-α expression.
Table 2.
Summary of various cytokine mRNAs and proteins detected by reverse transcriptase-polymerase chain reaction (RT-PCR) and ELISA
Effect of myoblast fusion and differentiation on the response to proinflammatory cytokines
When mononucleated myoblasts fuse to form multinucleated myotubes, muscle-specific gene expression is altered [10]. To determine whether the differentiation of myoblasts into myotubes has an effect on cytokine synthesis in response to proinflammatory stimuli, we compared the production of IL-6, TGF-β and GM-CSF in myoblasts and myotubes cultured under optimal concentrations of these stimuli. The secretion of IL-6 was higher in myoblasts than in myotubes under all conditions except after IFN-γ stimulation (Fig. 4a). TGF-β secretion was higher in myoblasts than in myotubes under all conditions of stimulation. IL-1β stimulation did not result in TGF-β synthesis either in myoblasts (see above) or in myotubes (Fig. 4b). GM-CSF secretion was higher in myoblasts than in myotubes under IL-1α and TNF-α stimulation. IL-1β induced less GM-CSF synthesis in myoblasts than in myotubes. Neither cell type secreted GM-CSF under IFN-γ stimulation (Fig. 4c).
Fig. 4.
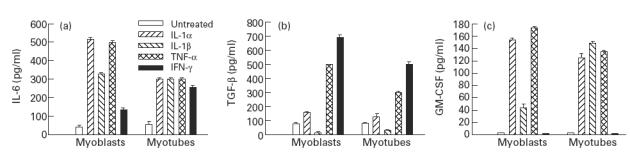
Comparison of the levels of synthesis of IL-6 (a), transforming growth factor-beta (TGF-β) (b) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (c) by myoblasts and myotubes after stimulation with IL-1α (50 ng/ml), IL-1β (100 ng/ml), tumour necrosis factor-alpha (TNF-α) (1 ng/ml) and IFN-γ (50 U/ml) for 48 h. The values are presented as mean (pg/ml) ± s.e.m. of three determinations in a representative experiment.
Constitutive and cytokine- and chemokine-stimulated expression of HLA class I, class II and ICAM-1 on muscle cells
Normal human skeletal myoblasts constitutively express very low levels of HLA class I. The proinflammatory cytokines at biologically active doses (IL-1α 1 ng/ml, IL-1β 1 ng/ml, TNF-α 1 ng/ml, and IFN-γ 500 U/ml) increased HLA class I expression on myoblasts, whereas TGF-β reduced the basal levels of HLA class I (Fig. 5). IL-6 did not show any effect on class I expression (data not shown). In the absence of added cytokines, HLA class II and ICAM-1 expression was not detected. IFN-γ induced high levels of class II expression, whereas other cytokines induced very low levels of HLA class II expression. All the cytokines with the exception of TGF-β induced significantly higher levels of ICAM-1 expression on myoblasts (Fig. 5).
Fig. 5.
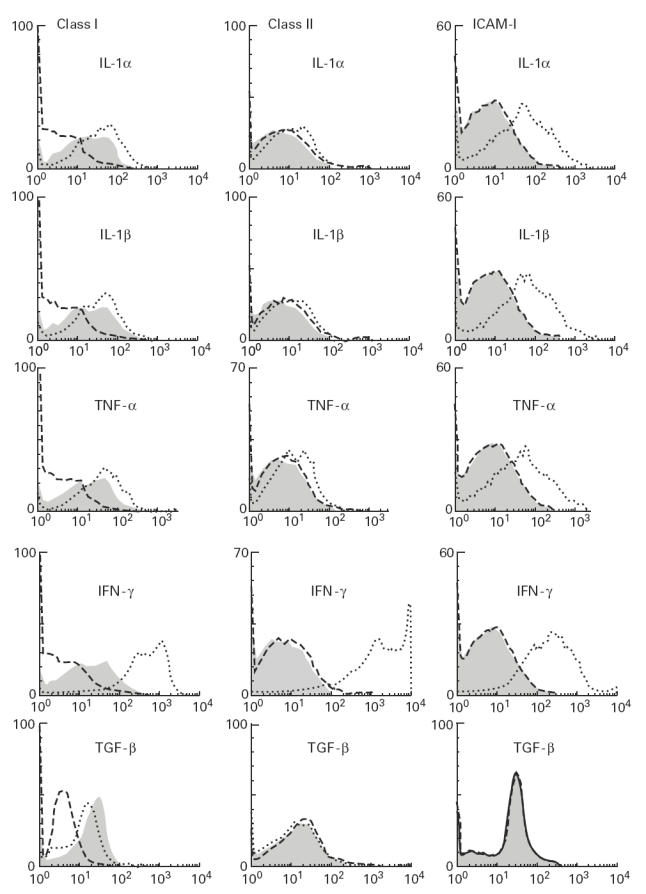
Flow cytometric analysis of myoblasts for HLA class I, HLA class II and intercellular adhesion molecule-1 (ICAM-1) after stimulation with IL-1α (1 ng/ml), IL-1β (1 ng/ml), tumour necrosis factor-alpha (TNF-α) (1 ng/ml), IFN-γ (500 U/ml) and transforming growth factor-beta (TGF-β) (10 ng/ml) for 48 h. Dashed lines, shaded areas, and dotted lines represent isotype-matched controls, unstimulated myoblasts, and stimulated myoblasts, respectively.
Recent experiments have demonstrated that mRNA for chemokines MIP-1α and RANTES are more commonly present in muscle biopsy specimens of patients with inflammatory myopathies than are any of the proinflammatory cytokines [11]. Therefore, we looked at the effects of various chemokines (MIP-1α, MIP-1β, MCP-1, RANTES, IL-8 and Gro-α) on muscle cells. Of these chemokines only MIP-1α induced low levels of HLA class I expression (Fig. 6e). Neither HLA class II nor ICAM-1 expression were affected by the chemokines (Fig. 6a–d).
Fig. 6.
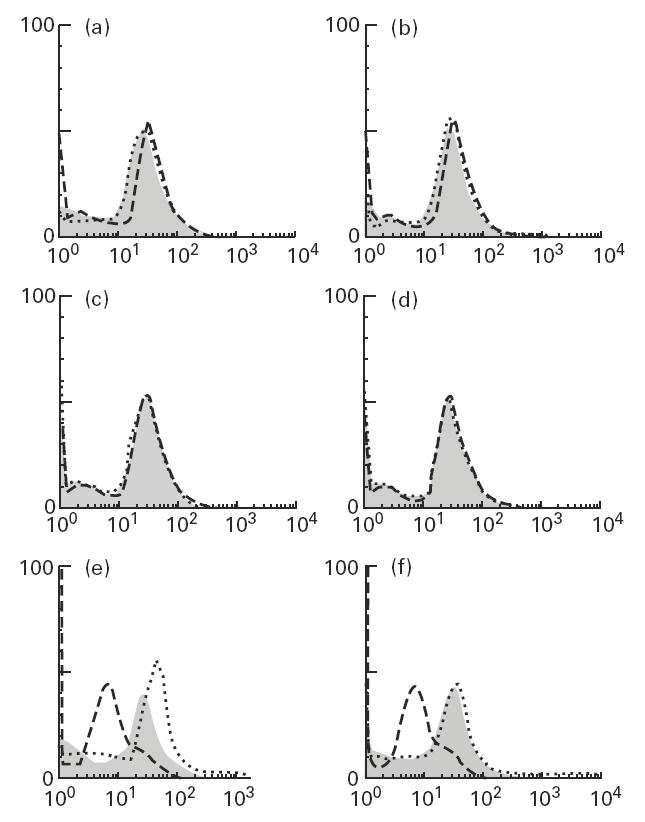
Flow cytometric analysis of myoblasts for HLA class I (e,f), HLA class II (c,d) and intercellular adhesion molecule-1 (ICAM-1) (a,b) after stimulation with MIP-1α (a,c,e) (100 ng/ml) and MCP-1 (b,d,f) (100 ng/ml) for 48 h. Dashed lines, shaded areas, and dotted lines represent isotype-matched controls, untreated myoblasts, and stimulated myoblasts, respectively.
Cytokines IL-1α, IL-1β, TNF-α, IFN-γ and IL-6, and chemokines IL-8, MIP-1α, MIP-1β, RANTES, IL-8, Gro-α and MCP-1 were used to determine whether the differentiation of myoblasts into myotubes has any effect on HLA class I expression. HLA class I was induced in myotubes by the same set of cytokines, which induced class I expression in myoblasts. In myotubes, as in myoblasts, MIP-1α stimulated low levels of HLA class I expression (Fig. 7).
Fig. 7.
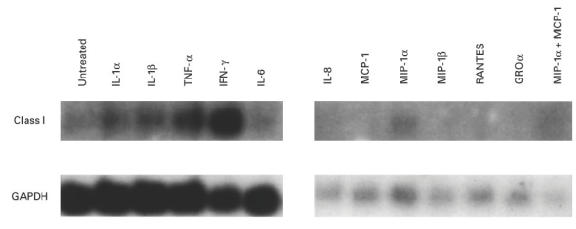
Northern hybridization for the detection of HLA class I on myotubes after stimulation with IL-1α (1 ng/ml), IL-1β (1 ng/ml), tumour necrosis factor-alpha (TNF-α) (1 ng/ml), IFN-γ (500 U/ml), IL-6 (10 ng/ml) and chemokines IL-8 (100 ng/ml), MCP-1 (100 ng/ml), MIP-1α (100 ng/ml), MIP-1β (100 ng/ml), RANTES (1 ng/ml), GRO-α (100 ng/ml) and combination of MIP-1α and MCP-1 for 48 h.
DISCUSSION
Infiltration of skeletal muscle by inflammatory cells has been reported in a number of chronic pathologic conditions, including autoimmune inflammatory myopathies, muscular dystrophies, post-poliomyelitis progressive muscular atrophy, and Graves' disease [12–14]. The cells are usually activated T cells, B cells, macrophages, and occasionally natural killer (NK) cells [15–17]. These activated immune cells are capable of secreting a variety of cytokines, and in fact various cytokines and chemokines have been found in muscle biopsies of inflammatory myopathies by a variety of techniques [11,18,19]. The source and the effects of these local cytokines, however, are not known.
In the studies described here, we sought to determine whether the proinflammatory stimuli induce the secretion of other proinflammatory cytokines by muscle cells themselves, and whether these stimuli also up-regulate the immunologically important surface antigens HLA class I and II and ICAM-1 on muscle cells. Although all of the experiments were performed with a fetal cell line, the changes observed are consistent with many observations on adult muscle biopsies [17–19].
We show that normal human muscle cells in vitro constitutively express IL-6 and TGF-β, suggesting that these cytokines have a role in muscle homeostasis. Indeed, IL-6 and TGF-β have been shown to induce neuronal differentiation and to inhibit myogenesis [20]. The proinflammatory cytokines increased secretion of both IL-6, which may help differentiation of B cells and activation of T cells in the inflammatory milieu [21], and TGF-β, which has the opposite effect [20]. The increased TGF-β levels may promote fibrosis by stimulating the synthesis of extracellular matrix [22]. In addition to IL-6 and TGF-β, cultured human myoblasts and myotubes secreted GM-CSF under inflammatory conditions.
The synthesis of mRNAs for IFN-α, TNF-α, MIP-1α and IL-1β was also detected, but the meaning of this finding is unclear, since secreted proteins were not detected. They may be translated but remain cell-bound, or they may be secreted below the level of detection. Under no test conditions did we detect any IL-1α, IL-4, IL-10, IL-12 or IFN-γ synthesis by human skeletal myoblasts.
There are limited data regarding the actual detection of cytokine proteins secreted by muscle cells. One report showed the constitutive and TNF-α- or/and IFN-γ-induced expression of IL-6 by human myoblasts [23]. In our study, we also detected the presence of TGF-β and GM-CSF proteins in culture supernatants. The cytokine proteins for IFN-α, TNF-α, MIP-1α were not detected by ELISA even after 10-fold concentration of culture supernatants.
The effects of proinflammatory stimuli varied over time of exposure in culture, and differed with different stimuli. Thus, although both TNF-α and IFN-γ caused early IL-6 secretion, the generally suppressive TGF-β peaked early (9 h) after IFN-γ, but much later (48 h) after TNF-α. In rheumatoid arthritis, TNF-α appears to be at the apex of the proinflammatory cytokine cascade, and blockade of TNF-α reduced the levels of other proinflammatory cytokines such as IL-1 and IL-6 [24]. In this study, TNF-α was found to induce high levels of IL-6, TGF-β and GM-CSF as early as 3 h, suggesting that it may have a similar position in muscle inflammation.
Although professional APC are required for the generation of an antigen-specific immune response, cells not normally capable of antigen presentation may acquire HLA class I, class II and ICAM-1 along with co-stimulatory molecules after induction by inflammatory cytokines such as IFN-γ [25]. Thus, facultative APC-like activated muscle cells may be responsible for increasing immune-mediated inflammation.
Although proinflammatory stimuli such as IFN-γ and TNF-α have previously been shown to induce expression of HLA class I and II antigens and ICAM-1 on muscle cells [26–29], IFN-γ and TNF-α are often absent in the biopsy samples of patients with inflammatory myopathies. Therefore, we looked to other possible cytokines and chemokines for their ability to induce expression of HLA class I, class II and ICAM-1. We found that not only do other proinflammatory cytokines such as IL-1α and IL-1β induce class I and ICAM-1 cell surface expression, but the C-C chemokine MIP-1α also induces class I expression, albeit weakly. TGF-β reduced class I expression. It is possible that the constitutive expression and local autocrine effect of TGF-β may be among the factors responsible for the very low expression of HLA class I on muscle cells.
Thus, muscle cells have an inherent ability to express and respond to a variety of cytokines and chemokines, depending on the inflammatory stimuli, and the apparently important up-regulation of HLA class I, class II and ICAM-1 in inflamed muscle may be influenced by the secreted products of the stimulation. Such interactions may be responsible for the perpetuation of autoimmune inflammatory myopathies and contribute to the rejection of transplanted myoblasts.
Acknowledgments
We thank Dr Iain Mcinnes for his helpful suggestions and comments.
References
- 1.Streilein JW. Peripheral tolerance induction: lessons from immune privileged sites and tissues. Transplant Proc. 1996;28:2066–70. [PubMed] [Google Scholar]
- 2.Law PK, Goodwin TG, Fang Q, et al. Human gene therapy with myoblast transfer. Transplant Proc. 1997;29:2234–7. doi: 10.1016/s0041-1345(97)00312-6. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Bagarazzi ML, Trivedi N, et al. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat Biotechnol. 1997;15:641–6. doi: 10.1038/nbt0797-641. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg NL, Ringel SP, Kotzin BL. Experimental autoimmune myositis in SJL/J mice. Clin Exp Immunol. 1987;68:117–29. [PMC free article] [PubMed] [Google Scholar]
- 5.Plotz PH, Miller FW. Animal models of myositis. Mt Sinai J Med (NY) 1988;55:501–5. [PubMed] [Google Scholar]
- 6.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of mouse transformining growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura T, Tange T, Terasawa T, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989;140:323–34. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- 8.Nordan RP, Pumphrey JG, Rudikoff S. Purification and NH2-terminal sequence of a plasmacytoma growth factor derived from the murine macrophage cell line P388D1. J Immunol. 1987;139:813–7. [PubMed] [Google Scholar]
- 9.Assoian RK, Fleurdelys BE, Stevenson HC, et al. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–4. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JB, Everitt EA, Smith TH, Block NE, Dominov JA. Cellular and molecular diversity in skeletal muscle development: news from in vitro and in vivo. Bioessays. 1993;15:191–6. doi: 10.1002/bies.950150308. [DOI] [PubMed] [Google Scholar]
- 11.Adams EM, Kirkley J, Eidelman G, Dohlman J, Plotz PH. The predominance of beta (CC) chemokine transcripts in idiopathic inflammatory muscle diseases. Proc Assoc Am Physicians. 1997;109:275–85. [PubMed] [Google Scholar]
- 12.Behan WM, Behan PO, Durward WF, McQueen A. The inflammatory process in polymyositis: monoclonal antibody analysis of muscle and peripheral blood immunoregulatory lymphocytes. J Neurol Neurosurg Psychiatry. 1987;50:1468–74. doi: 10.1136/jnnp.50.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figarella-Branger D, Pellissier JF, Bianco N, Devictor B, Toga M. Inflammatory and non-inflammatory inclusion body myositis. Characterization of the mononuclear cells and expression of the immunoreactive class I major histocompatibility complex product. Acta Neuropathol (Berl) 1990;79:528–36. doi: 10.1007/BF00296113. [DOI] [PubMed] [Google Scholar]
- 14.Zuk JA, Fletcher A. Skeletal muscle expression of class II histocompatibility antigens (HLA-DR) in polymyositis and other muscle disorders with an inflammatory infiltrate. J Clin Pathol. 1988;41:410–4. doi: 10.1136/jcp.41.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel AG, Arahata K. Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986;17:704–21. doi: 10.1016/s0046-8177(86)80180-0. [DOI] [PubMed] [Google Scholar]
- 16.Karpati G, Pouliot Y, Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol. 1988;23:64–72. doi: 10.1002/ana.410230111. [DOI] [PubMed] [Google Scholar]
- 17.Emslie-Smith AM, Arahata K, Engel AG. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol. 1989;20:224–31. doi: 10.1016/0046-8177(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 18.Lundberg I, Brengman JM, Engel AG. Analysis of cytokine expression in muscle in inflammatory myopathies, Duchenne dystrophy, and non-weak controls. J Neuroimmunol. 1995;63:9–16. doi: 10.1016/0165-5728(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 19.Tews DS, Goebel HH. Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol. 1996;55:342–7. doi: 10.1097/00005072-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 21.Randall TD, Lund FE, Brewer JW, Aldridge C, Wall R, Corley RB. Interleukin-5 (IL-5) and IL-6 define two molecularly distinct pathways of B-cell differentiation. Mol Cell Biol. 1993;13:3929–36. doi: 10.1128/mcb.13.7.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel AG, Hohlfeld R, Banker BQ. The polymyositis and dermatomyositis syndromes. In: Engel AG, Franzini-Armstrong C, editors. Myology. New York: McGraw Hill, Inc.; 1994. pp. 1335–83. [Google Scholar]
- 23.Bartoccioni E, Gallucci S, Scuderi F, et al. MHC class I, MHC class II and intercellular adhesion molecule-1 (ICAM-1) expression in inflammatory myopathies. Clin Exp Immunol. 1994;95:166–72. doi: 10.1111/j.1365-2249.1994.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 25.Tews DS, Goebel HH. Expression of cell adhesion molecules in inflammatory myopathies. J Neuroimmunol. 1995;59:185–94. doi: 10.1016/0165-5728(95)00045-4. [DOI] [PubMed] [Google Scholar]
- 26.Hohlfeld R, Engel AG. Induction of HLA-DR expression on human myoblasts with interferon-gamma. Am J Pathol. 1990;136:503–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Mantegazza R, Hughes SM, Mitchell D, Travis M, Blau HM, Steinman L. Modulation of MHC class II antigen expression in human myoblasts after treatment with IFN-gamma. Neurology. 1991;41:1128–32. doi: 10.1212/wnl.41.7.1128. [DOI] [PubMed] [Google Scholar]
- 28.Beauchamp JR, Abraham DJ, Bou-Gharios G, Partridge TA, Olsen I. Expression and function of heterotypic adhesion molecules during differentiation of human skeletal muscle in culture. Am J Pathol. 1992;140:387–401. [PMC free article] [PubMed] [Google Scholar]
- 29.Michaelis D, Goebels N, Hohlfeld R. Constitutive and cytokine-induced expression of human leukocyte antigens and cell adhesion molecules by human myotubes. Am J Pathol. 1993;143:1142–9. [PMC free article] [PubMed] [Google Scholar]


