Fig. 3.
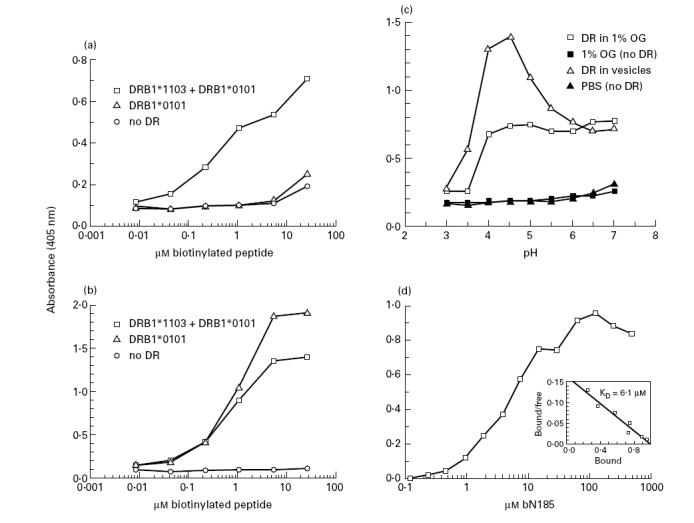
Optimal binding between the N185 and DRB1*1103 protein at acidic pH. Biotinylated peptide and DR samples (10 nm) were incubated under various conditions for 2 days at 37°C. Biotinylated peptide–DR complexes were then captured and revealed in wells of microtitre plates. (a,b) Increasing concentrations of bN185 and bF254 peptide were incubated with DR material in 1% n-octyl β-d-glucopyranoside isolated from 6396 B cells (DRB1*1103 + DRB1*0101) or LG-2 B cells (DRB1*0101) or without DR (no DR) at pH 4.5. (a) bN185 peptide, (b) bF254 peptide, (c) bN185 peptide (16 μm) were incubated with or without purified DRB1*1103 samples either solubilized in 1% n-octyl β-d-glucopyranoside or in lipid vesicles in 100 mm citrate-phosphate buffer at the indicated pH. (d) Increasing concentrations of bN185 peptide were incubated at pH 4.5 with DRB1*1103 samples reconstituted in lipid vesicles. The KD value for the bN185–DRB1*1103 complexes was calculated by Scatchard analysis (d, insert). Values are means of duplicates. Variations were < 15% of the mean. Results are representative of at least three independent experiments.
