Abstract
In the present study we describe the in vitro transcription-translation of human melanocyte-specific protein Pmel17 cDNA and subsequent use of the resulting 35S-labelled Pmel17 in an RIA to analyse vitiligo sera for the presence of Pmel17 antibodies. Of 53 vitiligo sera examined in the assay, three (5.9%) were found to be positive for Pmel17 antibodies. In contrast, sera from 20 healthy controls, 10 patients with Hashimoto's thyroiditis and 10 patients with Graves' disease (GD) were all negative for Pmel17 antibodies. All three patients positive for Pmel17 antibodies (aged 50–63 years) had had vitiligo of the symmetrical type for > 1 year and all of them also had an associated autoimmune disorder: GD in one and autoimmune hypothyroidism in two. In addition, all three patients had antibodies to the melanogenic enzymes tyrosinase, tyrosinase-related protein-1 (TRP-1) and tyrosinase-related protein-2 (TRP-2) in their serum. Absorption studies indicated that preincubation with COS-7 cell extract containing expressed Pmel17 absorbed out the immunoreactivity of the three sera positive in the RIA, confirming the anti-Pmel17 reactivity of the sera from these patients. In contrast, COS-7 cell extracts containing either expressed tyrosinase, TRP-1 or TRP-2 did not remove the anti-Pmel17 reactivity of the three sera in the RIA. This lack of cross-reactivity suggests that the humoral response to Pmel17 in these patients is specific and independent of the antibody reactivity to tyrosinase, TRP-1 and TRP-2.
Keywords: autoantigen, autoimmunity, vitiligo, Pmel17, gp100
INTRODUCTION
Vitiligo is a common skin disorder characterized by areas of depigmentation resulting from loss of melanocytes in the epidermis. Although the precise aetiology remains obscure, some studies have shown that anti-melanocyte antibodies are often present in the sera of vitiligo patients [1] and that there is a correlation between the presence and level of these antibodies and the extent [2] and activity of vitiligo [3]. In addition, the sera from vitiligo patients can induce damage to human melanocytes in vitro by antibody-dependent cellular cytotoxicity [4]. These findings suggest that anti-melanocyte antibodies may be involved in disease pathogenesis, although it is also possible that antibody production may merely reflect a secondary immunological response to melanocytes damaged by other mechanisms.
Recent work has tried to identify the antigens against which vitiligo antibodies react. Studies have shown that a number of pigment cell antigens can be immunoprecipitated with vitiligo sera [5]. These antigens are located on the cell surface, with some being preferentially expressed on pigment cells and others appearing to be common tissue antigens. Tyrosinase [6–8] and tyrosinase-related protein-2 (TRP-2) [9] have been implicated as autoantigens in vitiligo. Tyrosinase-related protein-1 (TRP-1) has not been recognized as an antigen against which human vitiligo sera react in certain studies [6,10], but we have found TRP-1 antibodies to be present in some vitiligo patients [11] and the TRP-1 protein has been implicated as an autoantigen in Smyth line chickens which express a genetically inherited form of vitiligo-like depigmentation [12].
Pmel17 is a melanosomal matrix glycoprotein [13,14] whose expression is melanocyte-specific and correlates closely with cellular melanin content [15,16]. The protein is encoded by the D12S53E gene which is the human homologue of the mouse silver (si) locus [17,18]. A limited degree of amino acid similarity exists among human Pmel17, tyrosinase and TRP-1 in five short regions of the three proteins [15,17]. In contrast, Pmel17 has extensive homology with chicken melanosomal matrix protein MMP115 [19], there being an overall amino acid identity between the two proteins of approx. 43% [15]. Pmel17 has also recently been identified as an important antigen on the surface of melanoma tumour cells recognized by cytotoxic T lymphocytes [20] and is variously referred to as gp100 [21,22] and ME20 [23].
In the present study we aimed to analyse vitiligo sera for the presence of antibodies to Pmel17 using an RIA with 35S-labelled recombinant human Pmel17. This type of assay is sensitive, quantitative, allows the detection of conformational epitopes and has been widely used to identify various autoantigens [8,9,24–26].
PATIENTS AND METHODS
Patients
Sera from 53 sequential vitiligo patients (16 men, 37 women; mean age 49 years; age range 18–79 years) collected in dermatology and endocrinology clinics between January 1990 and September 1996 were used in this study. Patients were characterized with respect to the presence of associated autoimmune diseases: 22 had no other disease and no family history of autoimmune disease; 12 had no other disease but had a family history of autoimmune disease; and 19 had an autoimmune disorder. The autoimmune diseases were: Graves' disease (GD), n = 4; autoimmune hypothyroidism, n = 9; alopecia areata, n = 2; Addison's disease with autoimmune hypothyroidism and type 1 diabetes mellitus, n = 1; autoimmune hypothyroidism and pernicious anaemia, n = 1; and type 1 diabetes mellitus, n = 2.
Sera from 20 healthy laboratory personnel, with no history of either vitiligo or any autoimmune disorder (nine men, 11 women; age range 23–47 years; mean age 31 years), were used as controls. As a further two sets of controls, 10 sera from patients (one man, nine women; age range 30–74 years; mean age 51 years) with Hashimoto's thyroiditis (HT) and 10 sera from patients (three men, seven women; age range 27–66 years; mean age 42 years) with GD were tested.
All sera were kept frozen at −20°C. The study was approved by the Ethics Committee of the Northern General Hospital, Sheffield, and all subjects gave informed consent.
Antisera
Rabbit polyclonal antisera αPEP7 [27], generated against a synthetic peptide which corresponds to the carboxyl terminus of mouse tyrosinase, and αPEP8 [27], generated against a synthetic peptide which corresponds to the carboxyl terminus of mouse TRP-2, were a gift of Professor V. Hearing (National Institutes of Health, Bethesda, MD). Pmel17-specific rabbit polyclonal antiserum AZN-LAM [28] was a gift of Dr M. Schreurs (Department of Tumour Immunology, University Hospital Nijmegen, Nijmegen, The Netherlands).
In vitro coupled transcription-translation of human Pmel17
Full-length human Pmel17 cDNA in pcDNA3 (Invitrogen, Abingdon, UK) was a gift of Doctor P. Robbins (National Institutes of Health) and was in the correct orientation for expression of Pmel17 from the T7 promoter in the vector. The plasmid, pMel17, was used in a TnT T7 coupled reticulocyte lysate system (Promega, Southampton, UK) to produce and label Pmel17 with 35S-methionine in vitro. Briefly, 2 μg of pMel17 were incubated for 120 min at 30°C in a 50-μl reaction mixture containing 25 μl rabbit reticulocyte lysate, 1 μl T7 TnT RNA polymerase, 1 μl amino acids minus methionine, 40 U RNasin (Promega), 2 μl TnT reaction buffer and 4 μl translation-grade 35S-methionine (1000 Ci/mmol; 10 mCi/ml; Amersham, Aylesbury, UK). The percentage incorporation of 35S-methionine was determined by trichloroacetic acid (TCA) precipitation, as previously described [8], and the reaction was stored at −20°C until needed. In some experiments, 5 μl of canine microsomal membranes (Promega) were added to the reaction mixture in order to glycosylate Pmel17.
Electrophoretic analysis and autoradiography
SDS–PAGE of in vitro translated products was performed in 10% SDS–polyacrylamide resolving gels [8,29] which were stained, dried and autoradiographed as described elsewhere [8,29].
RIA for Pmel17 antibodies
For each assay, an aliquot of the in vitro translation reaction mixture (equivalent to 12 000–20 000 ct/min of TCA-precipitable material) was suspended in 50 μl of immunoprecipitation buffer containing 20 mm Tris–HCl pH 8.0, 150 mm NaCl, 1% Triton X-100 and 10 mg/ml aprotinin. Serum was then added to a final dilution of 1:10 unless stated otherwise. After incubation overnight with shaking at 4°C, 50 μl of protein G Sepharose 4 Fast Flow slurry (Pharmacia Biotech, Uppsala, Sweden), prepared according to the manufacturer's directions, were added and incubated for 1 h at 4°C. The protein G Sepharose–antibody complexes were then collected by centrifugation and washed six times for 15 min in immunoprecipitation buffer at 4°C. Immunoprecipitated radioactivity was evaluated in an LKB 1217 Rackbeta liquid scintillation analyser.
For analysis by SDS–PAGE and autoradiography, the protein G Sepharose–antibody complexes were resuspended in 100 μl of SDS sample buffer [8,29], boiled, centrifuged and the supernatant recovered for electrophoresis in a 10% SDS–polyacrylamide gel.
For dilution experiments, each positive vitiligo serum and six healthy control sera were used in the RIA at final dilutions of 1:23.2, 1:58, 1:116, 1:232, 1:580, 1:1160 and 1:2320.
Expression of Pmel17 antibody levels
Pmel17 antibody levels were expressed as a relative antibody index (Pmel17Ab index). A Pmel17Ab index for each serum tested in the RIA was calculated as: ct/min immunoprecipitated by tested serum divided by the mean ct/min immunoprecipitated by 20 healthy controls. Each serum was tested in three experiments and the mean Pmel17Ab index was calculated from these. The s.d. of the mean was always within 15%. The upper level of normal for the assay was calculated using the mean Pmel17Ab index + 3 s.d. of a population of 20 healthy individuals.
For dilution experiments, a Pmel17Ab index was calculated for each serum tested at each dilution as: ct/min immunoprecipitated by tested serum at each dilution divided by the mean ct/min immunoprecipitated by six healthy controls at each dilution.
Transfection of COS-7 cells
For absorption experiments, Pmel17, tyrosinase, TRP-1 and TRP-2 were transiently expressed in COS-7 cells (European Collection of Animal Cell Cultures, Salisbury, UK), from pMel17, pcDNA3TYR [9], pCMVDTS [30] and pcDNA3TRP1 [11], respectively, as described elsewhere [9].
Transfected cells were washed three times in PBS containing 137 mm NaCl, 2.7 mm KCl, 10.1 mm Na2HPO4 and 1.8 mm KH2PO4 pH 7.4. To lyse the cells, 1 ml of buffer containing 40 mm Tris–HCl pH 7.5, 1 mm EDTA, 150 mm NaCl, 1% NP-40, 10 μg/ml aprotinin (Bayer, Newbury, UK), 100 μm Nα-tosyl-phenylalanyl chloromethyl ketone (Novobiochem, Nottingham, UK), 100 μm Nα-tosyl-lysyl chloromethyl ketone (Sigma, Poole, UK) and 10 μm pepstatin A (Novobiochem) were added to each plate and left for 5 min at 4°C. Unlysed cells were removed by centrifugation at 12 000 g for 5 min at 4°C. Extracts of untransfected cells were made in the same way. The total protein content of the extracts was determined by the method of Bradford [31] and the extracts diluted where appropriate to contain equivalent amounts of total protein.
Absorption experiments
In absorption experiments, vitiligo sera positive for Pmel17 antibodies and six healthy controls were first incubated at 4°C for 16 h with COS-7 cell extract containing either expressed Pmel17, tyrosinase, TRP-1 or TRP-2 and with COS-7 cell extract as control. The extracts used contained equivalent amounts of total protein. After preincubation, in vitro translated 35S-Pmel17 was added and the RIA carried out as previously described. A Pmel17Ab index was calculated for each serum as: ct/min immunoprecipitated by tested serum divided by the mean ct/min immunoprecipitated by six healthy controls.
RESULTS
In vitro transcription-translation of recombinant human Pmel17 and immunoprecipitation of 35S-Pmel17
In vitro transcription-translation of pMel17 resulted in an incorporation of 35S-methionine into Pmel17 of 8.8 ± 0.83% (mean ± s.d.) in four separate experiments. The quality of the in vitro translated 35S-Pmel17 was evaluated by SDS–PAGE and autoradiography which revealed a protein product with an estimated molecular weight of 66 kD (Fig. 1a). This agrees well with the molecular weight of 68 kD predicted from the amino acid sequence of the protein [17]. On addition of canine microsomal membranes to the in vitro translation reaction, a second higher molecular weight band at 97 kD was visible after SDS–PAGE and autoradiography (Fig. 1a). We assume this to be glycosylated Pmel17.
Fig. 1.
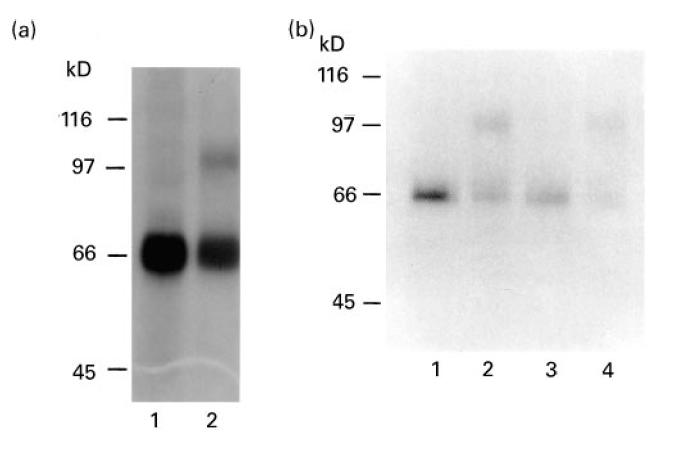
SDS–PAGE and autoradiography of in vitro translated human 35S-Pmel17. (a) 35S-Pmel17 (lane 1), 35S-Pmel17 produced in the presence of canine microsomal membranes (lane 2). (b) 35S-Pmel17 (lane 1), 35S-Pmel17 produced in the presence of canine microsomal membranes (lane 2), 35S-Pmel17 immunoprecipitated with Pmel17-specific AZN-LAM antiserum (lane 3), 35S-Pmel17 produced in the presence of canine microsomal membranes and immunoprecipitated with Pmel17-specific AZN-LAM (lane 4).
The immunoreactivity of the in vitro translated 35S-Pmel17 was tested using rabbit polyclonal antisera: both 35S-Pmel17 and the 97-kD protein were immunoprecipitated by Pmel17-specific AZN-LAM antiserum (Fig. 1b) but not by either tyrosinase-specific αPEP7 antiserum or TRP-2-specific αPEP8 antiserum (data not shown).
RIA of patient and control sera
Sera from 53 vitiligo patients, 20 healthy controls, 10 HT patients and 10 patients with GD were tested for their ability to immunoprecipitate 35S-Pmel17. For each serum a Pmel17Ab index was assigned, this being the mean Pmel17Ab index of three experiments with a s.d. of < 15%. The upper level of normal for the RIA (mean Pmel17Ab index + 3 s.d. of 20 healthy controls) was estimated as a Pmel17Ab index of 1.17 (Fig. 2).
Fig. 2.
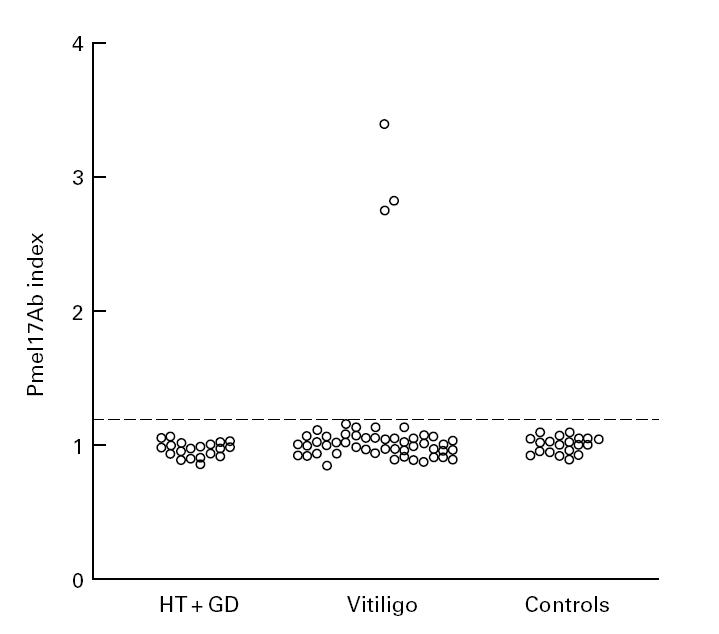
Pmel17Ab index of vitiligo patient sera (n = 53), Hashimoto's thyroiditis (HT) patient sera (n = 10), Graves' disease (GD) patient sera (n = 10) and healthy control sera (n = 20). The Pmel17Ab index shown for each serum is the mean of three experiments, and the s.d. of the mean was always within 15%. The dotted line shows the upper level of normal of 1.17 (mean Pmel17Ab index + 3 s.d. of 20 healthy controls) for the RIA.
None of the healthy individuals was positive for Pmel17 antibodies. From 53 vitiligo patients, three (5.9%) had a Pmel17Ab index above 1.17 (Fig. 2) and were considered positive for Pmel17 antibodies. Sera from 10 patients with HT and 10 patients with GD had a mean Pmel17Ab index of 0.96 ± 0.06 (mean ± s.d.) and 0.98 ± 0.06 (mean ± s.d.), respectively. All 20 sera were negative for antibodies to Pmel17. When used in the RIA, the translation products made in the presence of canine microsomal membranes, and thereby containing 35S-Pmel17 and the 97-kD protein, did not alter the Pmel17Ab index of any of the sera tested.
SDS–PAGE and autoradiography were used to check that the radioactivity immunoprecipitated by each of the positive sera was due to 35S-Pmel17. All Pmel17 antibody-positive sera immunoprecipitated a band of the correct size when compared with 35S-Pmel17 (Fig. 3a). In addition, all positive sera immunoprecipitated both 35S-Pmel17 and the 97-kD protein (Fig. 3b).
Fig. 3.
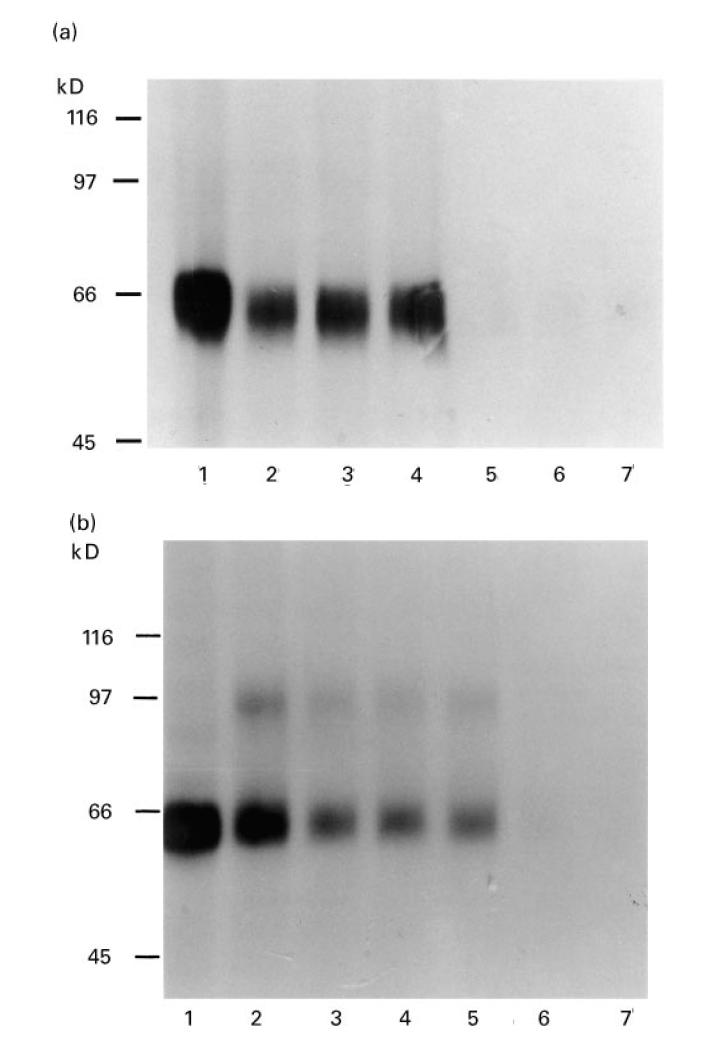
SDS–PAGE and autoradiography of 35S-Pmel17 immunoprecipitated with either vitiligo or healthy sera. (a) 35S-Pmel17 (lane 1), 35S-Pmel17 immunoprecipitated with Pmel17 antibody-positive vitiligo sera (lanes 2–4), with Pmel17 antibody-negative vitiligo sera (lanes 5 and 6) and with a healthy control serum (lane 7). (b) 35S-Pmel17 (lane 1), 35S-Pmel17 produced in the presence of canine microsomal membranes (lane 2), 35S-Pmel17 produced in the presence of canine microsomal membranes and immunoprecipitated with Pmel17 antibody-positive vitiligo sera (lanes 3–5), with a Pmel17 antibody-negative vitiligo serum (lane 6) and with a healthy control serum (lane 7).
The three Pmel17 antibody-positive sera were analysed at different dilutions in the RIA along with a group of six healthy controls. A Pmel17Ab index for each serum sample at each dilution was plotted as a function of 1/serum dilution (Fig. 4). For all three sera, saturated binding was observed at dilutions up to 1:100.
Fig. 4.
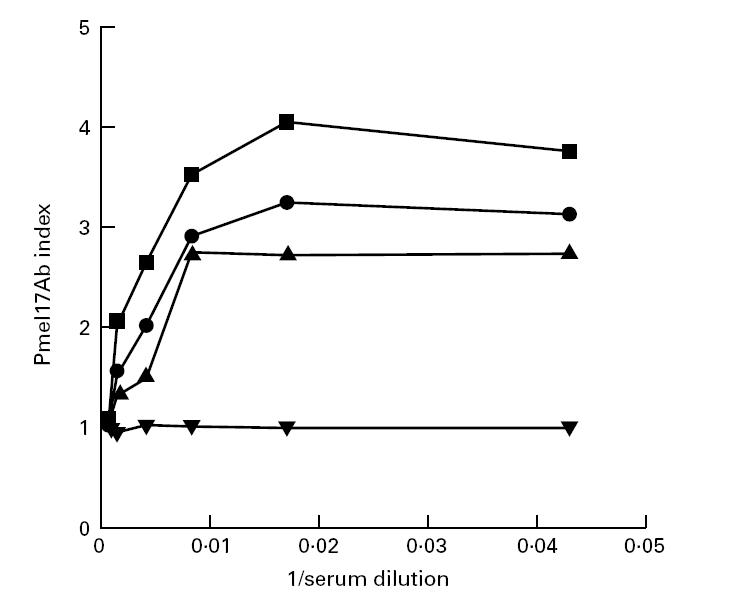
Dilution curves of Pmel17 antibody-positive sera and one healthy control serum. The Pmel17Ab index of each positive serum and one healthy control serum at each dilution is plotted as a function of 1/serum dilution. Each Pmel17Ab index is the mean Pmel17Ab index of three experiments with a s.d. within 15%. ▪, Pmel17 antibody-positive serum 1; •, Pmel17 antibody-positive serum 2; ▴, Pmel17 antibody-positive serum 3; ▾, healthy control serum.
Absorption experiments with vitiligo patient sera
Absorption experiments were carried out to assay any cross-reactivity of the Pmel17 antibodies with either tyrosinase, TRP-1 or TRP-2. Positive vitiligo sera reacting with 35S-Pmel17 in the RIA were preabsorbed with COS-7 cell extracts containing either expressed Pmel17, tyrosinase, TRP-2 or TRP-1 and with control COS-7 cell extract. The subsequent immunoprecipitation using in vitro translated 35S-Pmel17 showed that the anti-Pmel17 reactivity was greatly decreased in the serum samples preabsorbed with COS-7 cell extract which contained expressed Pmel17 (Fig. 5). In contrast, the binding ability of the serum samples preincubated with control COS-7 cell extract and extracts containing either expressed tyrosinase, TRP-2 or TRP-1 was not affected (Fig. 5).
Fig. 5.
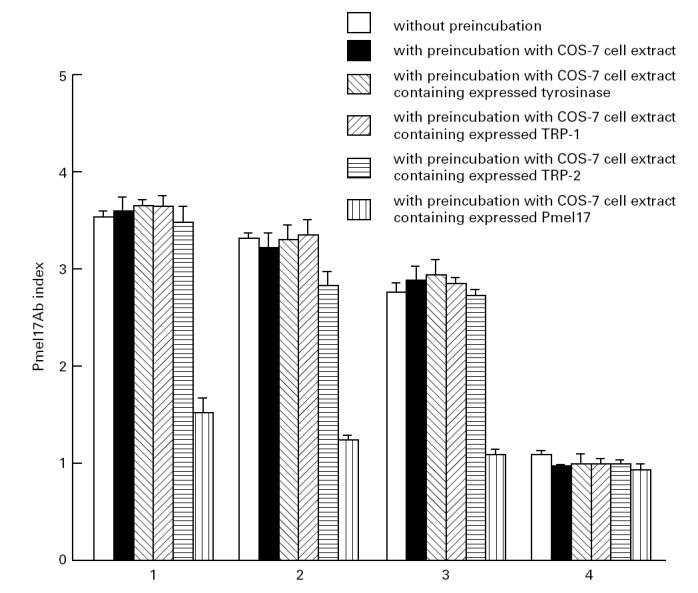
Absorption experiments with vitiligo patient sera positive for Pmel17 antibodies. The results of three experiments are shown as the mean Pmel17Ab index and s.d. for the three Pmel17 antibody-positive sera (1–3) and one control serum (4) without preincubation, with preincubation with COS-7 cell extract, with preincubation with COS-7 cell extract containing expressed tyrosinase, with preincubation with COS-7 cell extract containing expressed TRP-1, with preincubation with COS-7 cell extract containing expressed TRP-2 and with preincubation with COS-7 cell extract containing expressed Pmel17.
DISCUSSION
Recently, RIAs have been developed to detect specific antibodies in the sera of patients with autoimmune disease [8,9,24–26]. These involve in vitro translation and concomitant radiolabelling of the antigen of interest. The method combines sensitivity with the possibility of detecting conformational epitopes which may be of more importance in the pathogenesis of autoimmune disorders than reactivities detected by immunoblotting. It also allows a quantitative measurement of the level of antibodies and avoids the need to express and purify the protein of interest from either bacterial or mammalian cells. We describe here the use of an RIA to detect Pmel17 antibodies in sera from patients with vitiligo.
Using coupled in vitro transcription-translation we were able to produce 35S-labelled recombinant human Pmel17. This was of high quality as evaluated by SDS–PAGE and contamination by lower molecular weight products was minimal. On addition of canine microsomal membranes to the in vitro translation reaction, a second higher molecular weight band at 97 kD was visible after SDS–PAGE and autoradiography. We assume this to be glycosylated Pmel17. Both 35S-Pmel17 and the 97-kD protein were immunoreactive and could be immunoprecipitated by specific anti-Pmel17 antiserum.
The RIA was used to examine vitiligo and control sera for Pmel17 antibodies. Healthy control sera and sera from patients with HT or GD did not contain Pmel17 antibodies. From 53 vitiligo sera tested, three (5.9%) contained Pmel17 antibodies. When used in the RIA, the translation products made in the presence of canine microsomal membranes, and thereby containing 35S-Pmel17 and the 97-kD protein, did not alter the Pmel17Ab index of any of the sera tested, suggesting that any Pmel17 epitope recognized by the three positive sera was not altered by glycosylating the protein and that the Pmel17 antibody-negative vitiligo sera did not recognize glycosylated epitopes or epitopes altered by post-translational processing of the protein.
All three patients (aged 50–63 years) positive for Pmel17 antibodies had had vitiligo of the symmetrical type for > 1 year and all of them also had an associated autoimmune disorder: GD in one and autoimmune hypothyroidism in two. In addition, all three patients had antibodies to the melanogenic enzymes tyrosinase [8,9], TRP-2 [9] and TRP-1 [11] in their serum. Since a limited degree of amino acid similarity exists among human Pmel17, tyrosinase and TRP-1 in five short regions of the three proteins [15,17], we used absorption experiments to determine if the Pmel17 antibodies, detected in the three vitiligo patients, cross-reacted with the other melanogenic proteins. The immunoreactivity of the three positive sera in the RIA could be preabsorbed with COS-7 cell extract containing expressed Pmel17, thereby confirming the anti-Pmel17 reactivity of the sera. In contrast, COS-7 cell extracts containing either expressed tyrosinase, TRP-1 or TRP-2, did not remove the anti-Pmel17 reactivity of the three sera in the RIA. These three antigen preparations have been shown previously to be effective in absorbing out their respective autoantibodies [8,9,11]. This lack of cross-reactivity suggests that the humoral response to Pmel17 in these patients is specific and independent of the antibody reactivity to tyrosinase, TRP-1 and TRP-2.
We do not yet know if the humoral autoimmune response to this antigen in these patients is important in the initial onset of vitiligo or if it is provoked by destruction of melanocytes by another mechanism with the subsequent release of autoantigen protein. However, the results indicate that Pmel17 antibodies only occur at a low frequency in vitiligo sera and that Pmel17 does not appear to be a major antigen in patients with this disorder. Given the high frequency of antibodies in vitiligo sera [1], it seems likely that melanocyte autoantigens other than Pmel17 are important targets of the autoimmune response in vitiligo, but it is striking that there appears to be an association between the presence of autoantibodies to tyrosinase, TRP-1, TRP-2 and Pmel17 in our patients, suggesting that this combination of autoantibodies may define a particular subset of vitiligo patients. Furthermore, the Pmel17 antibodies detected in vitiligo patient sera in this study may be of interest in the treatment of melanoma, as Pmel17 is a tumour antigen recognized by cytotoxic T lymphocytes [20] and vitiligo autoantibodies have been shown to have a destructive effect on melanoma cells in vitro and in vivo [32].
Acknowledgments
This work was supported by a grant from the Special Trustees for the Former United Sheffield Hospitals' Charitable Funds (grant no. 87758). We would like to thank Professor Vincent Hearing for antisera αPEP7 and αPEP8, Dr Paul Robbins for Pmel17 cDNA, Dr Marco Schreurs for Pmel17-specific rabbit polyclonal antiserum AZN-LAM, Professor Shigeki Shibahara (Tohoku University School of Medicine, Sendai, Japan) for tyrosinase, TRP-1 and TRP-2 cDNAs and Mrs Janine Phipps for growing COS-7 cells.
References
- 1.Naughton GK, Eisinger M, Bystryn JC. Detection of antibodies to melanocytes in vitiligo by specific immunoprecipitation. J Invest Dermatol. 1983;81:540–2. doi: 10.1111/1523-1747.ep12522891. [DOI] [PubMed] [Google Scholar]
- 2.Naughton GK, Reggiardo D, Bystryn JC. Correlation between vitiligo antibodies and extent of depigmentation in vitiligo. J Am Acad Dermatol. 1986;15:978–81. doi: 10.1016/s0190-9622(86)70260-0. [DOI] [PubMed] [Google Scholar]
- 3.Harning R, Cui J, Bystryn JC. Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol. 1991;97:1078–80. doi: 10.1111/1523-1747.ep12492607. [DOI] [PubMed] [Google Scholar]
- 4.Norris DA, Kissinger RM, Naughton GK, Bystryn JC. Evidence for immunologic mechanisms in human vitiligo: patients' sera induce damage to human melanocytes in vitro by complement-mediated damage and antibody-dependent cellular cytotoxicity (ADDC) J Invest Dermatol. 1988;90:783–9. doi: 10.1111/1523-1747.ep12461505. [DOI] [PubMed] [Google Scholar]
- 5.Cui J, Harning R, Henn M, Bystryn JC. Identification of pigment cell antigens defined by vitiligo antibodies. J Invest Dermatol. 1992;98:162–5. doi: 10.1111/1523-1747.ep12555773. [DOI] [PubMed] [Google Scholar]
- 6.Song YH, Connor E, Li Y, Zorovich B, Balducci P, Maclaren N. The role of tyrosinase in autoimmune vitiligo. Lancet. 1994;344:1049–52. doi: 10.1016/s0140-6736(94)91709-4. [DOI] [PubMed] [Google Scholar]
- 7.Baharav E, Merimski O, Shoenfeld Y, Zigelman R, Gilbrund B, Yecheskel G. Tyrosinase as an autoantigen in patients with vitiligo. Clin Exp Immunol. 1996;105:84–88. doi: 10.1046/j.1365-2249.1996.d01-727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemp EH, Gawkrodger DJ, MacNeil S, Watson PF, Weetman AP. Detection of tyrosinase autoantibodies in vitiligo patients using 35S-labelled recombinant human tyrosinase in a radioimmunoassay. J Invest Dermatol. 1997;109:69–73. doi: 10.1111/1523-1747.ep12276556. [DOI] [PubMed] [Google Scholar]
- 9.Kemp EH, Gawkrodger DJ, Watson PF, Weetman AP. Immunoprecipitation of melanogenic enzyme autoantigens with vitiligo sera: evidence for cross-reactive autoantibodies to tyrosinase and tyrosinase-related protein-2 (TRP-2) Clin Exp Immunol. 1997;109:495–500. doi: 10.1046/j.1365-2249.1997.4781381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Arita Y, Bystryn JC. Characterisation of vitiligo antigens. Pigment Cell Res. 1995;8:53–59. doi: 10.1111/j.1600-0749.1995.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 11.Kemp EH, Waterman EA, Gawkrodger DJ, Watson PF, Weetman AP. Autoantibodies to tyrosinase-related protein-1 (TRP-1) detected in the sera of vitiligo patients using a quantitative radiobinding assay. Brit J Dermatol. 1998 doi: 10.1046/j.1365-2133.1998.02503.x. in press. [DOI] [PubMed] [Google Scholar]
- 12.Austin LM, Boissy RE. Mammalian tyrosinase-related protein-1 is recognised by autoantibodies from vitiliginous Smyth chickens. Am J Pathol. 1995;146:1529–41. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B-K, Kobayashi T, Donatien PD, Bennett DC, Hearing VJ, Orlow SJ. Identification of a melanosomal matrix protein encoded by the murine si (silver) locus using ‘organelle scanning’. Proc Natl Acad Sci USA. 1994;91:7076–80. doi: 10.1073/pnas.91.15.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi T, Urabe K, Orlow SJ, Higashi K, Imokawa G, Kwon BS, Potter B, Hearing VJ. The Pmel17/Silver locus protein. Characterisation and investigation of its melanogenic function. J Biol Chem. 1994;269:29198–205. [PubMed] [Google Scholar]
- 15.Kwon BS. Pigmentation genes: the tyrosinase gene family and the pmel 17 gene family. J Invest Dermatol. 1993;100:134S–40S. doi: 10.1111/1523-1747.ep12465022. [DOI] [PubMed] [Google Scholar]
- 16.Kwon BS, Halaban R, Kim GS, Usack L, Pomerantz SH, Haq AK. A melanocyte-specific cDNA clone whose expression is inducible by MSH and IBMX. Mol Biol Med. 1987;4:339–55. [PubMed] [Google Scholar]
- 17.Kwon BS, Chintamaneni C, Kozak CA, et al. A melanocyte-specific gene, Pmel17, maps near the silver coat color locus on mouse chromosome 10 and is in a syntenic region on human chromosome 12. Proc Natl Acad Sci USA. 1991;88:9228–32. doi: 10.1073/pnas.88.20.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailin T, Lee S-T, Spritz RA. Genomic organisation and sequence of D12S53E (Pmel17), the human homologue of the mouse silver (si) locus. J Invest Dermatol. 1996;106:24–27. doi: 10.1111/1523-1747.ep12326976. [DOI] [PubMed] [Google Scholar]
- 19.Mochii M, Agata K, Eguchi G. Complete sequence and expression of a cDNA encoding 115-kD melanosomal matrix protein. Pigment Cell Res. 1991;4:41–47. doi: 10.1111/j.1600-0749.1991.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 20.Bakker ABH, Scheurs MWJ, de Boer AJ, Kawakami Y, Rosenberg SA, Adema GJ, Figdor CG. Melanocyte lineage-specific antigen gp100 is recognised by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–9. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adema GJ, de Boer AJ, Vogel AM, Loenen WAM, Figdor CG. Molecular characterisation of the melanocyte lineage-specific antigen gp100. J Biol Chem. 1994;269:20126–33. [PubMed] [Google Scholar]
- 22.Kawakami Y, Eliyahu S, Delgado CH, et al. Identification of a human melanoma antigen recognised by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458–62. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maresh GA, Marken JS, Neubauer M, Aruffo A, Hellstrom I, Hellstrom KE, Marquardt H. Cloning and expression of the gene for the melanoma-associated ME20 antigen. DNA Cell Biol. 1994;13:87–95. doi: 10.1089/dna.1994.13.87. [DOI] [PubMed] [Google Scholar]
- 24.Falorni A, Grubin CE, Takei I, et al. Radioimmunoassay detects the frequent occurrence of autoantibodies to the Mr 65,000 isoform of glutamic acid decarboxylase in Japanese insulin-dependent diabetes. Autoimmunity. 1994;19:113–25. doi: 10.3109/08916939409009539. [DOI] [PubMed] [Google Scholar]
- 25.Falorni A, Nikoshokov A, Laureti S, et al. High diagnostic accuracy for idiopathic Addison's disease with a sensitive radiobinding assay for autoantibodies against recombinant human 21-hydroxylase. J Clin Endocrinol Metab. 1995;80:2752–5. doi: 10.1210/jcem.80.9.7673419. [DOI] [PubMed] [Google Scholar]
- 26.Peterson P, Perheentupa J, Krohn KJE. Detection of candidal antigens in autoimmune polyglandular syndrome type I. Clin Diag Lab Immunol. 1996;3:290–4. doi: 10.1128/cdli.3.3.290-294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukamoto K, Jackson IJ, Urabe K, Montague PM, Hearing VJ. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992;11:519–26. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreurs MJW, de Boer AJ, Schmidt A, Figdor CG, Adema GJ. Cloning, expression and tissue distribution of the murine homologue of the melanocyte lineage-specific antigen gp100. Melanoma Res. 1997;7:463–70. doi: 10.1097/00008390-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch EF, Maniatis T. A laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. Molecular cloning. [Google Scholar]
- 30.Yokoyama K, Suzuki H, Yasumoto K, Tomita Y, Shibahara S. Molecular cloning and functional analysis of cDNA coding for human DOPAchrome tautomerase/tyrosinase-related protein-2. Biochim Biophys Acta. 1994;1217:317–21. doi: 10.1016/0167-4781(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 32.Fishman P, Aziz E, Shoenfeld Y, et al. Vitiligo autoantibodies are effective against melanoma. Cancer. 1993;72:2365–9. doi: 10.1002/1097-0142(19931015)72:8<2365::aid-cncr2820720812>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


