SUMMARY
Neonates have a significant requirement for cholesterol. From −1 to 25 days of age, the liver accrues 6.9 mg cholesterol and the extra-hepatic tissues accrue 107.7 mg cholesterol in the hamster. It is currently unknown if each of these body compartments synthesizes their own cholesterol or if they have alternative source(s) of sterol. Using 3H2O, in vivo hepatic sterol synthesis rates (per g liver per animal) increased between −1 and 5 days of age, decreased by 10 days of age, and increased again by 15 days of age. HMG-CoA reductase (HMGR) expression levels paralleled in vivo synthesis rates. Extra-hepatic sterol synthesis rates followed the same pattern as sterol synthesis rates in the liver. When sterol synthesis rates were converted to the mass of sterol synthesized per day, the liver synthesized 38.9 and the extra-hepatic tissues synthesized 63.9 mg cholesterol in the 26-day neonatal period. Comparing the amount of cholesterol accrued to that synthesized, one can conclude that the liver is a major source of sterol for the whole body during the neonatal period of the hamster. These results may help elucidate the cause(s) of reduced growth rates in neonates with liver disease or in neonates with compromised sterol synthesis rates.
Keywords: development, cholesterol, Smith-Lemli-Opitz, cholestasis, HMG-CoA reductase, neonate
INTRODUCTION
The requirement for cholesterol varies among animals of different ages. Healthy adults are in steady state and are not growing. Their sole requirement for cholesterol is to compensate for the net loss of sterol that occurs from conversion of sterols to bile acids and hormones, from loss of secreted biliary cholesterol, and from sloughed cells [1]. In contrast, embryos, fetuses, and neonates are not in steady state and have a rapid growth rate. For every kg of tissue that is added to the body of the younger animals, a minimum of 1.5–2.0 g of cholesterol is needed for membrane formation alone [1]. In the central nervous system, ≈20 mg/g of cholesterol is needed to support growth [1, 2]. In addition to the cholesterol required for membrane formation in these younger animals, cholesterol is also required to compensate for the net loss of sterols due to bile acid and hormone synthesis, loss of biliary cholesterol, and sloughed cells. When cholesterol is lacking, substrates essential for membrane formation would be inadequate and growth rates may be affected.
It has been difficult to determine the role of cholesterol in neonatal and childhood growth rates due to compensatory changes in sterol synthesis rates with the addition or deletion of dietary cholesterol [3, 4]. One group of individuals that could be used to assess the role of cholesterol in growth rates due to their inability to synthesize cholesterol at normal rates are patients with the Smith-Lemli-Opitz syndrome (SLOS) [5, 6]. As might be expected in persons unable to synthesize cholesterol, growth rates of SLOS infants and children are often markedly reduced [7]. Interestingly, impaired growth rates of SLOS patients are improved when diets are supplemented with cholesterol [8–10]. Thus, it does appear that an increase in available cholesterol may impact upon growth rates under certain conditions.
There are two sources of cholesterol in the body of younger and older individuals alike. Cholesterol can be obtained from the diet and cholesterol can be synthesized de novo. All tissues synthesize cholesterol, though the rates vary with type of tissue, animal species, and age [1]. In adult humans and hamsters, the contribution of cholesterol synthesized in the liver is much less than that synthesized in the remainder of the body [1]. While several studies have demonstrated that hepatic sterol synthesis rates are greater in younger versus older animals [1, 11–14], the relative amount of sterol synthesized in the liver versus the remainder of the body is unknown in the rapidly growing neonates.
Why is it important to know if tissues synthesize their own cholesterol or if they obtain cholesterol from other sources during neonatal development? If the liver synthesizes a significant amount of the cholesterol accrued by the whole body and cholesterol is needed for growth, then a decrease in hepatic sterol synthesis rates could lead to poor growth rates, as occurs in pediatric patients with SLOS or with liver disease. Thus, the purpose of the current studies was to delineate the origin of cholesterol utilized by the liver and the remainder of the body for membrane formation as well as other basic functions. To complete these studies, 3H2O was used to measure sterol synthesis rates in vivo [15]. This method is unique since the absolute mass of cholesterol synthesized per day can be determined. The hamster was used as the animal model since the rates of synthesis are relatively low in the liver as compared to the remainder of the body as occurs in humans [1] and since a positive sterol balance does not enhance bile acid synthesis rates, a phenomenon also observed in humans [16, 17]. The liver of the neonatal hamster synthesized ≈5-fold more cholesterol than the amount of sterol accrued whereas the extra-hepatic tissues synthesized ≈60% of that accrued. Thus, the liver synthesizes enough sterol to support its own requirement for cholesterol as well as some of the requirements in the remainder of the body.
MATERIALS AND METHODS
Animals and Diets
Non-pregnant female and male Golden Syrian hamsters weighing 90–100 g (Charles River, Inc., Kingston, NJ) were fed a pelleted chow diet with an inherent cholesterol concentration of 0.002% (wt/wt; 7102, Harlan Teklad, Madison, WI) and kept in a temperature and humidity controlled room with alternating light and darkness (14 h light/10 h dark). Female hamsters were mated as described previously [18]. Neonates had free access to food and water. One male and one female pup per litter were studied in the early dark light cycle at −1, 5, 10, 15, 20, and 25 days of age; tissues and plasmas from several pups at −1 day of age were pooled to generate one sample per litter. Adults were studied at 90 days after birth. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Plasma, Liver, and Carcass Cholesterol Concentrations
Animals were anesthetized, exsanguinated, and liver and carcass collected. Plasma cholesterol concentrations were measured enzymatically (Roche Diagnostics Corporation, Indianapolis, IN). Plasma was pooled and lipoproteins separated by FPLC [19]. Cholesterol in each fraction was measured enzymatically. A piece of liver and the remainder of the body (extra-hepatic tissues/carcass) were saponified and sterol was extracted. Pieces of liver were saponified in 5 ml of alcoholic KOH. Carcasses were saponified in 100–500 ml alcoholic KOH, and sterol was extracted from 3–10% of the saponified tissue. Tissue cholesterol concentrations were determined by gas liquid chromatography using stigmastanol as internal standard [20].
In Vivo Sterol and Fatty Acid Synthesis Rates
Adult and neonatal hamsters were injected ip with 5–20 mCi 3H2O. Pregnant dams were injected with 50 mCi 3H2O. After 1 h, animals were anesthetized and exsanguinated. Livers were removed from the body. Pieces of liver and the remainder of the body were saponified in alcoholic KOH as described in the previous section. Similar amounts of sample were used to measure sterol synthesis rates in duplicate. Sterol was extracted and digitonin-precipitable sterols (DPS) were isolated and assayed for 3H content [15]. Samples were subsequently acidified, fatty acids extracted, and extracts were assayed for 3H content. The rates of synthesis are presented as nmol 3H2O incorporated into sterol or fatty acids per h per g tissue or per tissue [15]; fetal synthesis rates were corrected for equilibration of 3H2O between the pregnant dam and the fetus [21, 22]. Synthesis rates are also presented as mg cholesterol synthesized per day per tissue taking into account the fact that 1.45 μg-atoms of carbon enter the cholesterol molecule from acetyl CoA for each μg-atom of 3H that is found in DPS [15, 23, 24]. Sterol synthesized during the study (1 h) was multiplied by 24 h to determine mg sterol synthesized per day since hamsters eat throughout the day [25, 26] and sterol synthesis rates parallel food consumption [27].
HMG CoA Reductase (HMGR) Expression Levels
Livers were collected and microsomes isolated as described [28]. Protein concentrations were determined by the Lowry assay [29] and equal amounts of protein from each litter at each age were pooled. Proteins were separated by SDS-PAGE using a Bio-Rad 4–15% Tris-HCl Ready gel (Biorad Laboratories, Hercules, CA) under denaturing conditions and then transferred to a PVDF membrane. The membrane was blocked in Tris-buffered saline containing 0.1% Tween and 5% dry milk and then incubated with rabbit anti-HMGR IgG (Upstate Cell Signaling Solutions, Lake Placid, NY). After 1 h at 37ºC, the membrane was incubated with a secondary antibody conjugated with peroxidase (donkey anti-rabbit IgG; GE Healthcare, Little Chalfont Buckinghamshire, UK). Chemiluminescence from ECL Plus (GE Healthcare) was detected by a Storm 450 Phosphoimager. Relative densities were determined using NIH Image J software. Blots were stripped with Restore Western Blot Stripping Buffer and reprobed with anti β-actin IgG (Millipore, Bilerica, MA).
Statistics
Data are presented as means ± SEM. Initially, males and females from each litter were analyzed separately. However, since no differences were observed between the sexes at these early ages, data from males and females were analyzed together. The effects of aging on various metabolic parameters were evaluated three different ways. First, data from sequential ages were compared to one another by t-tests. For example, −1 day was compared to 5 day, 5 day to 10 day, etc. Using this method, we could determine if values increased or decreased with age. Second, if there was no change in subsequent ages, due to slight differences, the slope of the line was calculated using least squares regression, where age was the independent variable. Significant slopes were significantly different from zero. Third, data from neonates at 25 days of age were compared to data from the adult males and the females by t-tests. Significance for all tests was set at P<0.05.
RESULTS
Neonatal hamsters have a rapid, exponential growth rate. The liver and whole body masses increased ≈25-fold in only 26 days (Fig. 1). The liver made up ≈4% of mass of the whole body throughout the whole neonatal period (Fig. 1A inset) and into adulthood (male-3.9±0.1%; female-3.9±0.2%).
Figure 1.
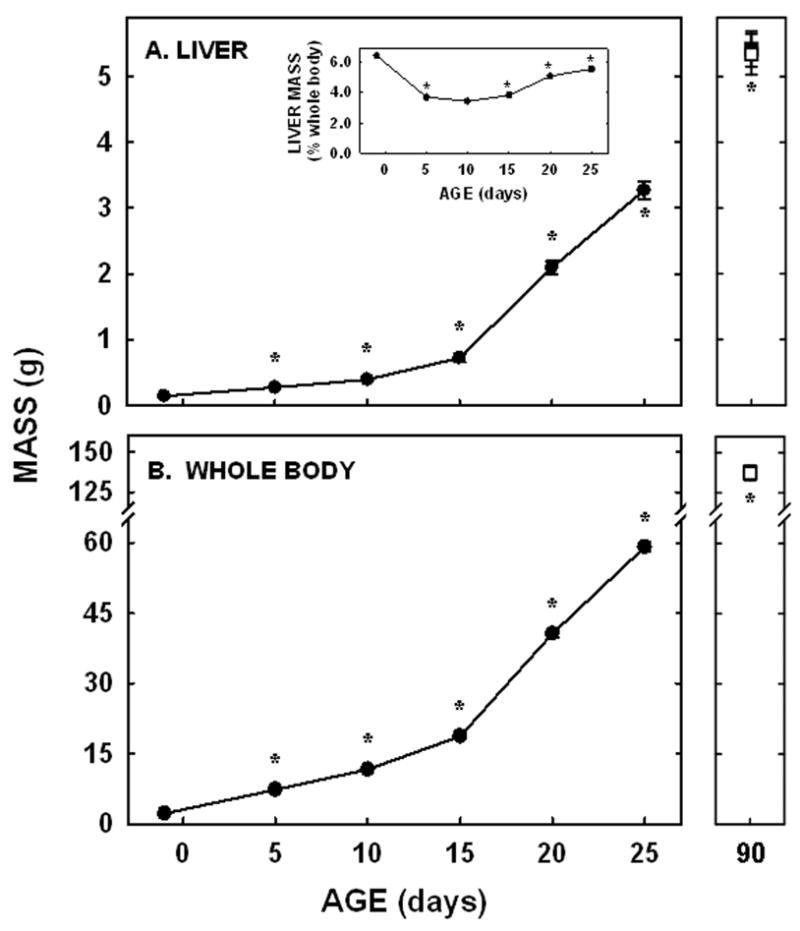
Whole body and liver masses in neonatal and adult hamsters. Liver (A) and whole body (B) weights were measured in neonates from −1 to 25 days of age and in adult male (closed square) and female (open square) hamsters. Data are presented as means ± SEM in 12–30 animals. * depicts significant differences (P<0.05) from values at the previous age. Slopes for body weight was 2.34±0.07 (P<0.001) and for liver weight was 0.12±0.01 (P<0.001).
Plasma cholesterol concentrations decreased between 1- and 5 days of age (P=0.003) and remained relatively constant until 20 days of age when concentrations increased again (P=0.02; Fig. 2A). There was no difference in concentrations between 25 and 90 days of age. A significant amount of plasma cholesterol was carried as VLDL/LDL-cholesterol at −1 day of age (Fig. 2A). Concentrations decreased by 5 days of age and remained fairly constant through to adulthood.
Figure 2.
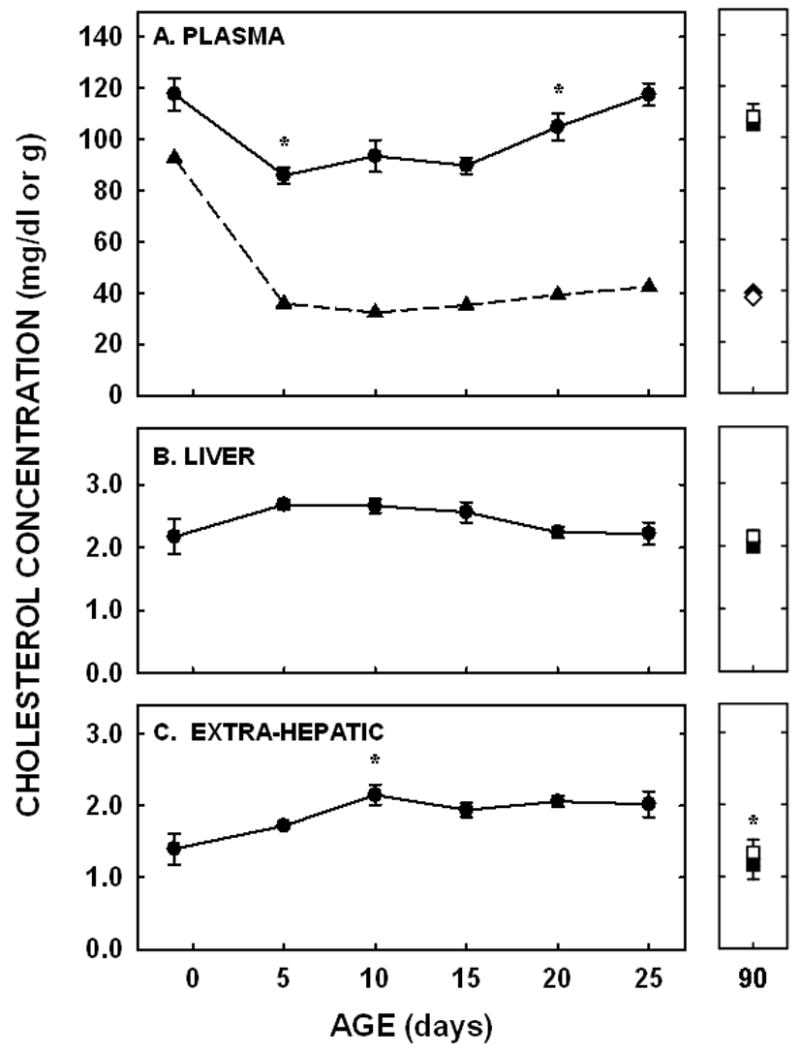
Plasma, VLDL/LDL, and liver cholesterol concentrations in neonatal and adult hamsters. Cholesterol concentrations were measured enzymatically in plasma (A; circles/squares) and VLDL/LDL FPLC fractions (A; triangles/diamonds) and by GLC in livers (B) and extra-hepatic tissues (C). Adult males were depicted by closed squares and diamonds and adult females by open squares and diamonds. Data are presented as means ± SEM in 3–18 animals (plasma), 4–30 animals (liver), or 7–15 animals (extra-hepatic tissues). Cholesterol contents in VLDL/LDL peaks were obtained from 3 pooled plasma samples. * depicts significant differences (P<0.05) from values at the previous age. Slopes for liver cholesterol concentration was −0.02±0.01 (P=0.022) and carcass cholesterol concentration was 0.02±0.01 (P=0.002).
Liver and extra-hepatic cholesterol concentrations did not follow the same pattern of change as did plasma cholesterol concentrations. In the liver, the cholesterol concentration was 2.17±0.28 mg/g at −1 day of age. There was little change in hepatic cholesterol concentrations between subsequent ages (Fig. 2B). A general decrease in cholesterol concentration did occur since the slope of the line for hepatic cholesterol concentrations throughout the neonatal period was significantly different from zero (P=0.02). By 25 days of age, concentrations were near adult values. In the remainder of the body, cholesterol concentrations increased between 5 and 10 days of age (P=0.01) and did not change significantly between subsequent ages throughout the remainder of the neonatal period (Fig. 2C). There was a general increase in cholesterol concentrations in the extra-hepatic tissues throughout the neonatal period, however, since the slope of the line from these data was also significantly different from zero (P=0.002). The extra-hepatic tissue cholesterol concentrations were lower in the adult males and females as compared to the 25 day old neonates (P<0.05). Taking into account cholesterol concentrations and tissue masses, the liver accrued 6.9 mg of cholesterol and the extra-hepatic tissues accrued 107.7 mg of cholesterol from −1 to 25 days of age.
To determine the amount of sterol synthesized in the liver and extra-hepatic tissues, in vivo sterol synthesis rates were measured using 3H2O. When presented as per g liver per animal, hepatic sterol synthesis rates increased between −1 and 5 days of age (P<0.001), decreased between 5 and 10 days of age (P=0.006), and increased again at 15 days of age (P=0.03) (Fig. 3A). Rates changed minimally between 15 and 25 days of age. By 90 days of age, synthesis rates had decreased in both male and female hamsters (P<0.05). Expression levels of HMGR were 12-, 1.4-, and 16-fold greater in the 5, 10, and 20 day old neonates as compared to the faint, but detectable band in the −1 day old neonate, respectively (Fig. 3C). Expression levels for adult livers were about 40% of the levels of the 20 day old neonates. Though β-actin was used as a control protein, these data may or may not be of physiological relevance since protein expression, and thus the proportion of various proteins within a tissue, varies markedly with maturation [12]. Since metabolism can be affected by body mass [1], hepatic sterol synthesis rates were normalized to a similar weight. When normalized to 100 g, hepatic sterol synthesis rates decreased with age (Fig. 3B). Interestingly, hepatic fatty acid synthesis rates did not parallel hepatic sterol synthesis rates in the neonatal period. Rates were very low at the early ages and increased ≈40-fold between 5 and 20 days of age (Fig. 4).
Figure 3.
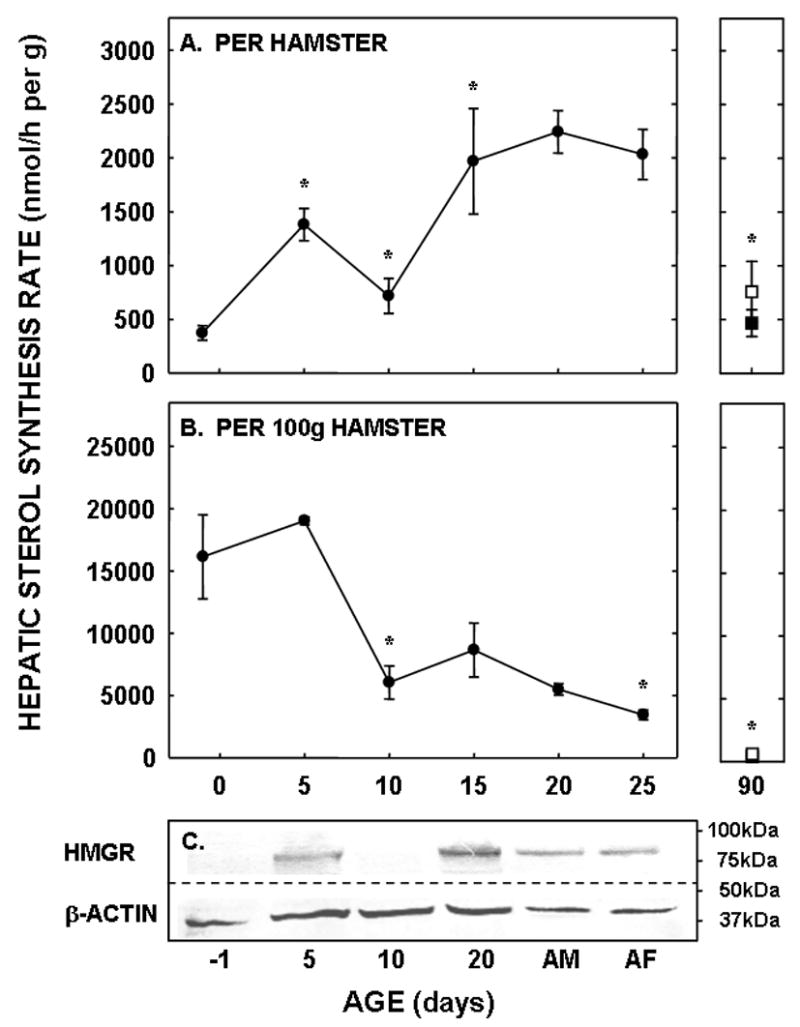
Hepatic sterol synthesis rates in neonatal and adult hamsters. Sterol synthesis rates were measured in vivo using 3H2O in neonates (−1 to 25 days of age) and adult males (closed square) and females (open square). Data are presented as nmol 3H2O converted to sterol per h per g liver per animal (A) and per g liver per 100 g animal (B). Data are presented as means ± SEM in 6–16 animals. In Panel C, the relative expression levels of HMGR and β-actin are shown in hamsters of increasing ages (AD-adult male, AF-adult female). * depicts significant differences (P<0.05) from values at the previous age. Slope for sterol synthesis rate per 100 g animal was −616.3±105.9 (P<0.001).
Figure 4.
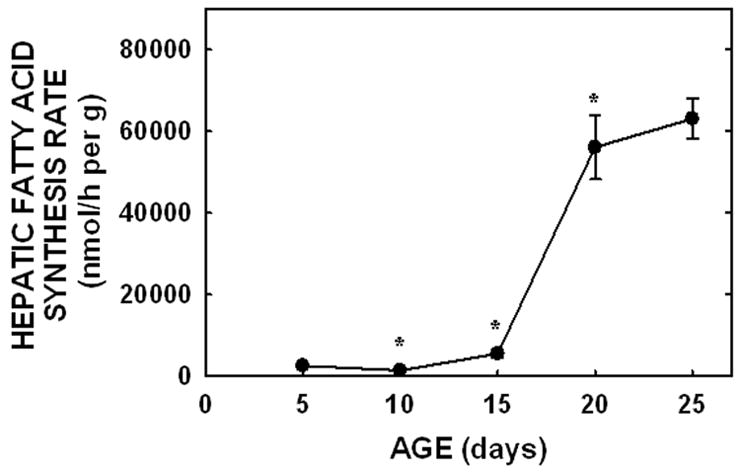
Hepatic fatty acid synthesis rates in neonatal hamsters. Fatty acid synthesis rates were measured in vivo using 3H2O in neonates (5 to 25 days of age). Data are presented as nmol 3H2O converted to fatty acid per h per g liver. Data are presented as means ± SEM in 10–30 animals. * depicts significant differences (P<0.05) from values at the previous age. Slope for fatty acid synthesis rate was 3438±336 (P<0.001).
Sterol synthesis rates (nmol per g tissue per animal) in the extra-hepatic tissues paralleled those in the liver in that there was an increase between −1 and 5 days of age (P=0.005), a decrease at 10 days of age (P<0.001), and an increase at 15 days of age (P=0.03) (Fig. 5A). Unlike the liver, synthesis rates in the extra-hepatic tissues decreased between 20 and 25 days of age (P<0.001). Rates were also lower in adult male and female hamsters as compared to male and female hamsters at 25 days of age (P<0.05). When presented as synthesis per g tissue in a 100 g animal, synthesis rates decreased continuously as depicted by a significant negative slope (P<0.001) (Fig. 5B).
Figure 5.
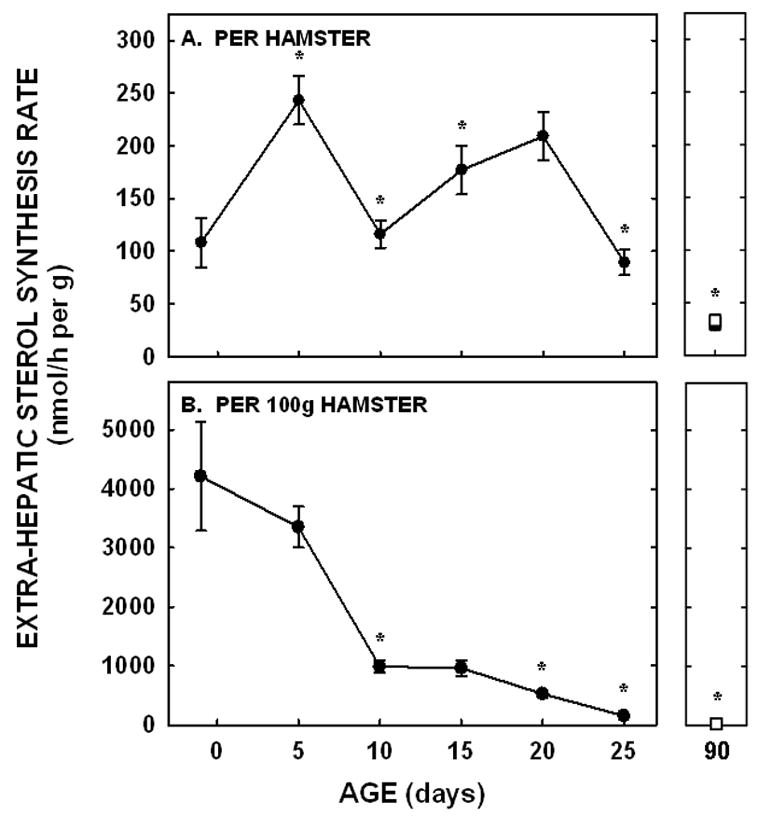
Extra-hepatic tissue sterol synthesis rates in neonatal and adult hamsters. Sterol synthesis rates were measured in vivo using 3H2O in neonates (−1 to 25 days of age) and adult males (closed squares) and females (open squares). Data are presented as nmol 3H2O converted to sterol per h per g tissue per animal (A) and per g tissue per 100 g animal (B). Data are presented as means ± SEM in 3–16 animals. * depicts significant differences (P<0.05) from values at the previous age. Slope for sterol synthesis rate per 100 g animal was −157.0±16.7 (P<0.001).
A fundamental reason for using 3H2O in these studies is that one can calculate the absolute mass of cholesterol synthesized per day. The sterol synthesis rates at each day of the 26 days presented in Figure 3A were changed from nmol water converted to sterol per h per g liver to mg cholesterol synthesized per day per whole liver [15]. The same was done for the synthesis rates in the extra-hepatic tissues presented in Fig. 5A. We then estimated the synthesis rates for the ages not directly measured from the plotted values at −1, 5, 10, 15, 20, and 25 days of age. By summing the mg of cholesterol synthesized per day, the cumulative sterol synthesis rates over the entire 26-day period was calculated, assuming a small margin of error since synthesis rates were not determined but estimated for some of the ages. We found that 38.9 mg of cholesterol was synthesized by the liver and 63.9 mg was synthesized by the extra-hepatic tissues.
DISCUSSION
During periods of rapid growth and cellular proliferation, a significant amount of cholesterol is required by all tissues for membrane formation as well as cellular function. In the liver of the hamster, 6.9 mg of cholesterol was accrued in neonates between -1 and 25 days of age. The remainder of the body accrued 107.7 mg of cholesterol during this same time period. Since 38.9 mg of cholesterol was synthesized by the liver and 63.9 mg was synthesized by the extra-hepatic tissues, the liver synthesized ≈5-fold more cholesterol than it needed for membrane formation alone whereas the remainder of the body synthesized ≈60% of the cholesterol it needed.
To determine if this difference between sterol accrued versus sterol synthesized occurred throughout the entire neonatal period, we determined the sterol accrued and the sterol synthesized during the slower (−1 to 15 days of age) and the more rapid (16 to 25 days of age) growth rates (Fig. 6). Regardless of whether or not growth rates were slower or more rapid, the liver synthesized more cholesterol than it accrued (Fig. 6A). There does appear to be a slight deficit of cholesterol synthesized versus that accrued in −1 to 15 days of life but not between days 16 and 25. There are two explanations for why more cholesterol was accrued as compared to that synthesized early in life. First, hamsters consume significant amounts of milk before 15 days of age [30]. Based on studies in rats [31] and taking into account the smaller size of the hamster, we assumed that a 10 day old hamster would consume ≈2 ml of milk per day. Since the cholesterol concentration of hamster milk is ≈55 mg/dl (L. Yao and L. Woollett, unpublished observation), the animals would consume ≈1.1 mg of cholesterol and absorb ≈0.5 mg of cholesterol per day. Though the younger animals would consume less milk, the cholesterol content of milk may be greater earlier in gestation [32] so significant amounts would still be consumed. This exogenous source of cholesterol could account for the extra cholesterol accrued as compared to that synthesized. It should be noted that even though more food was consumed with age, the proportion that was milk versus that which was chow is hard to determine since neonates begin to consume some solid food by 10 days of age and are self-weaned by 25 days of age [30]. Second, the gastrointestinal tract was not washed out prior to saponification of the carcass. Thus, the amount of cholesterol in the extra-hepatic tissues could have been slightly overestimated due to cholesterol-containing milk as well as biliary cholesterol in the gastrointestinal tract.
Figure 6.
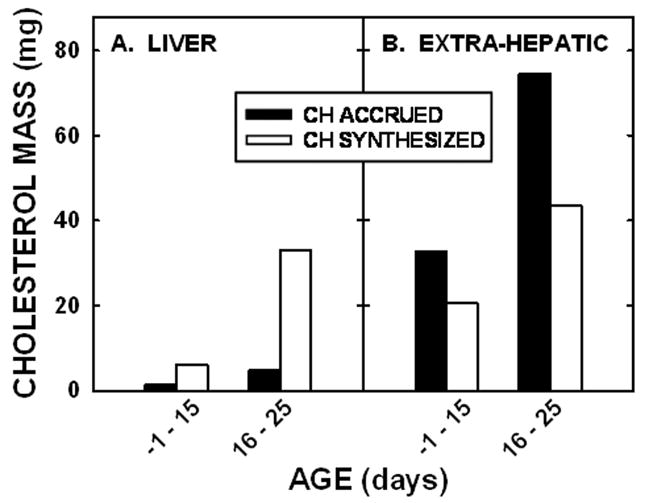
Net sterol balance in neonatal hamsters. The absolute masses of cholesterol synthesized and cholesterol accrued between −1 and 15 days of age or 16 to 25 days of age were calculated and presented for the liver (A) and extra-hepatic tissues (B) of neonatal hamsters.
Though the liver synthesized more cholesterol than it required, sterol synthesis rates were not continuously elevated from birth to weaning. Rates were elevated at 5 days of age, decreased between 5 and 10 days of age, and then increased again at 15 days of age, similar to enzyme activities measured previously in rats [12, 33, 34]. The consumption of significant amounts of dietary cholesterol could lead to the suppression of sterol synthesis rates and reduction in HMGR expression levels at 10 days of age, most likely through processing of the sterol regulatory element binding protein-2 (SREBP) [35, 36]. The increase in rate between 10 and 15 days of age is most likely due to the transition from consumption of a high fat, high cholesterol diet (milk) to a low fat, low cholesterol diet (chow).
Even taking into account the low synthesis rates at 10 days of age, the liver still synthesizes ≈5-fold more cholesterol than it needs to support its own tissue growth. During times of rapid tissue growth and cellular proliferation, such as in extra-embryonic and embryonic tissues or in transformed, malignant cells, there is a dysregulation of sterol synthesis and rates are constitutively elevated [37]–[38, 39]. We hypothesize that neonatal livers are in a negative sterol balance due to the high requirements of cholesterol needed for membrane production and bile acid synthesis as well as VLDL production, the route by which newly synthesized cholesterol is transported from the liver to all the peripheral tissues, excluding the CNS [40, 41]. We hypothesize that the elevated rates are maintained due to the continuous net loss of sterol (negative sterol balance). Sustained synthesis occurs during other experimental conditions or physiological states where the requirement for cholesterol is quite high and tissues are hypothesized to be in a negative sterol balance. First, animals fed bile acid sequestrants have a high requirement for cholesterol and hepatic sterol synthesis rates are elevated and sustained [42, 43]. Second, hepatic sterol synthesis rates are markedly elevated in mid-gestation [44, 45] when there is a net loss of sterol from the body as cholesterol-containing lipoproteins are taken up by the placenta and yolk sac [46]; the placenta and yolk sac are expelled from the body during parturition. Thus, though more studies are required to delineate the mechanism responsible for the markedly elevated hepatic sterol synthesis rates in the neonate, possible mechanisms include a net loss of hepatic cholesterol to be used in the production of VLDL or bile acids.
Two other interesting observations are made from the current studies. First, these in vivo results demonstrate that sterol and fatty acid synthesis rates are regulated differently during development. Sterol synthesis rates are elevated early in development (gestation and just after birth) whereas fatty acid synthesis rates increase mid/late into the suckling period. There are two transcription factors which are responsible for sterol and fatty acid synthesis, SREBP-2 and SREBP-1, respectively [36, 47–49]. Our data are consistent with the processing of SREBP-2 to the nuclear form earlier in development and the processing of SREBP-1 to the nuclear form later in development [50, 51]. Though both SREBPs require similar proteins for processing [35, 36], SREBP-1 may have additional transcriptional factors such as LXR and/or insulin [36, 52, 53], which are absent at different times during development [50, 51]. Second, these data illustrate the potential importance of post-translational modification of HMGR. As seen in panels A and C of Figure 3, the relative expression level of HMGR was greater in adult livers as compared to the −1 and 10 day old neonates whereas synthesis rates were similar. One situation where protein levels may not parallel activities is the phosphorylated state of the protein; though some controversy still exists in the field, it appears that HMGR activity is decreased when the protein is phosphorylated [54]. As one might then expect from our data, HMGR is more extensively phosphorylated in adults as compared to neonates [55], leading to a reduction in enzyme activity but not expression levels as seen in panels A and C of Figure 3.
What implications do these data for the neonatal human? These data would have the most relevance to individuals with impaired ability to synthesize cholesterol, such as patients with SLOS or other defects in sterol synthesis [7, 56]. Interestingly, the addition of cholesterol to the diet of growth-impaired SLOS infants improves their growth rates [8–10]. Supplementation of diet with cholesterol would not only increase the substrate supply for membranes, but also improve membrane function through modification of membrane fluidity [57–59]. In addition to the SLOS neonates, these data also have implications for neonates with cholestasis since hepatic sterol synthesis rates are decreased in individuals with cholestatic liver disease [60, 61]. While some of the infants with cholestasis, including Alagille syndrome, progressive familial intrahepatic cholestasis (PFIC I–III), neonatal hepatitis, and biliary atresia, have normal growth rates, others have growth retardation even within the same syndrome [62, 63]. Though the cause of the differences in growth is unknown, it is conventionally thought that failure to thrive is secondary to fat and fat soluble vitamin malabsorption [64, 65]. Based upon our findings, however, and the fact that some infants fail to thrive even when malabsorption is accounted for [62, 63], a decrease in the production of cholesterol by the affected liver might significantly reduce the amount of cholesterol presented to extra-hepatic tissues during times of rapid growth and therefore be a rate limiting factor for cellular proliferation. Thus, presenting exogenous cholesterol to infants/children with cholestasis might improve growth as in pediatric patients with SLOS.
Acknowledgments
The authors wish to thank Larly Bouquia and Julie McConihay for their excellent technical assistance. This work was supported by funding from grants HD34089 and HD39419 of the National Institutes of Health.
Abbreviations
- SLOS
Smith-Lemli-Opitz syndrome
- DPS
digitonin-precipitable sterols
- HMGR
HMG CoA Reductase
- SREBP
sterol regulatory element binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 2.Cavender CP, Turley SD, Dietschy JM. Sterol metabolism in fetal, newborn, and suckled lambs and their response to cholesterol after weaning. Am J Physiol. 1995;269:E331–E340. doi: 10.1152/ajpendo.1995.269.2.E331. [DOI] [PubMed] [Google Scholar]
- 3.Cruz MLA, Wong WW, Mimouni F, Hachey DL, Setchell KD, Klein PD, Tsang RC. Effects of infant nutrition on cholesterol synthesis rates. Pediatr Res. 1994;35:135–140. doi: 10.1203/00006450-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Bayley TM, Alasmi M, Thorkelson T, Krug-Wispe S, Hones PJH, Bulani JL, Tsang RC. Influence of formula versus breast milk on cholesterol synthesis rates in four-month-old infants. Pediatr Res. 1998;44:60–67. doi: 10.1203/00006450-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Irons M, Elias ER, Salen G, Tint GS, Batta AK. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- 6.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Eng J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 7.Porter FD. RSH/Smith-Lemli-Opitz Syndrome: A multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol Genet Metab. 2000;71:163–174. doi: 10.1006/mgme.2000.3069. [DOI] [PubMed] [Google Scholar]
- 8.Elias ER, Irons MB, Hurley AD, Tint GX, Salen G. Clinical effects of cholesterol supplementation in six patients with the Smith-Lemli-Opitz syndrome (SLOS) Am J Med Genet. 1997;68:305–310. doi: 10.1002/(sici)1096-8628(19970131)68:3<305::aid-ajmg11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Irons M, Elias ER, Abuelo D, Bull MJ, Greene CL, Johnson VP, Keppen L, Schanen C, Tint GS, Salen G. Treatment of Smith-Lemli-Opitz syndrome: results of a multi-center trial. Am J Med Genet. 1997;68:311–314. [PubMed] [Google Scholar]
- 10.Nwokoro NA, Mulvihill JJ. Cholesterol and bile acid replacement therapy in children and adults with Smith-Lemli-Opitz (SLO-RSH) syndrome. Am J Med Genet. 1997;68:315–321. doi: 10.1002/(sici)1096-8628(19970131)68:3<315::aid-ajmg13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Haave NC, Innis SM. Cholesterol synthesis and accretion within various tissues of the fetal and neonatal rat. Metabolism. 2001;50:12–18. doi: 10.1053/meta.2001.19498. [DOI] [PubMed] [Google Scholar]
- 12.Smith JL, Lear SR, Erickson SK. Developmental expression of elements of hepatic cholesterol metabolism in the rat. J Lipid Res. 1995;36:641–652. [PubMed] [Google Scholar]
- 13.Stange EF, Dietschy JM. Age-related decreases in tissue sterol acquisition are mediated by changes in cholesterol synthesis and not low density lipoprotein uptake in the rat. J Lipid Res. 1984;25:703–713. [PubMed] [Google Scholar]
- 14.Spady DK, Turley SD, Dietschy JM. Dissociation of hepatic cholesterol synthesis from hepatic low-density lipoprotein uptake and biliary cholesterol saturation in female and male hamsters of different ages. Biochim Biophys Acta. 1983;753:381–392. doi: 10.1016/0005-2760(83)90062-0. [DOI] [PubMed] [Google Scholar]
- 15.Dietschy JM, Spady DK. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984;25:1469–1476. [PubMed] [Google Scholar]
- 16.Horton JD, Cuthbert JA, Spady DK. Regulation of hepatic 7α-hydroxylase expression and response to dietary cholesterol in the rat and hamster. J Biol Chem. 1995;270:5381–5387. doi: 10.1074/jbc.270.10.5381. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi T, Chen J, Cooper AD. Regulation of cholesterol 7 alpha-hydroxylase gene expression in Hep-G2 cells. Effect of serum, bile salts, and coordinate and noncoordinate regulation with other sterol-responsive genes. J Biol Chem. 1994;269:10071–10078. [PubMed] [Google Scholar]
- 18.McConihay JA, Horn PS, Woollett LA. The effect of maternal hypercholesterolemia on fetal sterol metabolism in the Golden Syrian hamster. J Lipid Res. 2001;42:1111–1119. [PubMed] [Google Scholar]
- 19.deSilva HV, Mas-Oliva J, Taylor JM, Mahley RW. Identification of apolipoprotein B-100 low density lipoproteins, apolipoprotein B-48 remnants, and apolipoprotein E-rich high density lipoproteins in the mouse. J Lipid Res. 1994;35:1297–1310. [PubMed] [Google Scholar]
- 20.Turley SD, Herndon MW, Dietschy JM. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J Lipid Res. 1994;35:328–339. [PubMed] [Google Scholar]
- 21.Belknap WM, Dietschy JM. Sterol synthesis and low density lipoprotein clearance in vivo in the pregnant rat, placenta, and fetus. Sources for tissue cholesterol during fetal development. J Clin Invest. 1988;82:2077–2085. doi: 10.1172/JCI113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woollett LA. Origin of cholesterol in the fetal Golden Syrian hamster: contribution of de novo sterol synthesis and maternal-derived lipoprotein cholesterol. J Lipid Res. 1996;37:1246–1257. [PubMed] [Google Scholar]
- 23.Jeske DJ, Dietschy JM. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980;21:364–376. [PubMed] [Google Scholar]
- 24.Andersen JM, Dietschy JM. Absolute rates of cholesterol synthesis in extrahepatic tissues measured with 3H-labeled water and 14C-labeled substrates. J Lipid Res. 1979;20:740–752. [PubMed] [Google Scholar]
- 25.Zucker I, Stephen FK. Light-dark rhythms in hamster eating, drinking and locomotor behaviors. Physiol Behav. 1973;11:239–250. doi: 10.1016/0031-9384(73)90356-9. [DOI] [PubMed] [Google Scholar]
- 26.Silverman HJ, Zucker I. Absence of post-fast food compensation in golden hamster (Mesocrietus auratus) Physiol Behav. 1976;17:271–285. doi: 10.1016/0031-9384(76)90076-7. [DOI] [PubMed] [Google Scholar]
- 27.Andersen JM, Dietschy JM. Regulation of sterol synthesis in 16 tissues of rat. I. Effect of diurnal light cycling, fasting, stress, manipulation of enterohepatic circulation, and administration of chylomicrons and triton. J Biol Chem. 1977;252:3646–3651. [PubMed] [Google Scholar]
- 28.Jelinek DF, Andersson S, Slaughter CA, Russell DW. Cloning and regulation of cholesterol 7α-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J Biol Chem. 1990;265:8190–8197. [PMC free article] [PubMed] [Google Scholar]
- 29.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 30.Yao L, Woollett LA. Adult sterol metabolism is not affected by a positive sterol balance in the neonatal Golden Syrian hamster. Am J Physiol. 2005;288:R561–R566. doi: 10.1152/ajpregu.00353.2004. [DOI] [PubMed] [Google Scholar]
- 31.Fiorotto ML, Burrin DG, Perez M, Reeds PJ. Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am J Physiol. 1991;260:R1104–R1113. doi: 10.1152/ajpregu.1991.260.6.R1104. [DOI] [PubMed] [Google Scholar]
- 32.Harzer G, Haug M, Dieterich I, Gentner PR. Changing patterns of human milk lipids in the course of the lactation and during the day. Am J Clin Nutr. 1983;37:612–621. doi: 10.1093/ajcn/37.4.612. [DOI] [PubMed] [Google Scholar]
- 33.McNamara DJ, Quackenbush FW, Rodwell VW. Regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. Developmental pattern. J Biol Chem. 1972;247:5805–5810. [PubMed] [Google Scholar]
- 34.Haave NC, Innis SM. Perinatal development of hepatic cholesterol synthesis in the rat. Biochim Biophys Acta. 1991;1985:35–44. doi: 10.1016/0005-2760(91)90229-b. [DOI] [PubMed] [Google Scholar]
- 35.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid KE, Woollett LA. Differential effects of polyunsaturated fatty acids on sterol synthesis rates in adult and fetal tissues of the hamster: Consequence of altered sterol balance. Am J Physiol. 2003;285:G796–G803. doi: 10.1152/ajpgi.00226.2003. [DOI] [PubMed] [Google Scholar]
- 38.Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med. 2004;229:567–585. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- 39.Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21:505–517. [PubMed] [Google Scholar]
- 40.Turley SD, Burns DK, Dietschy JM. Preferential utilization of newly synthesized cholesterol for brain growth in neonatal lambs. Am J Physiol. 1998;274:E1099–E1105. doi: 10.1152/ajpendo.1998.274.6.E1099. [DOI] [PubMed] [Google Scholar]
- 41.Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Turley SD, Daggy BP, Dietschy JM. Psyllium augments the cholesterol-lowering action of cholestyramine in hamsters by enhancing sterol loss from the liver. Gastroenterology. 1994;107:444–452. doi: 10.1016/0016-5085(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 43.Horton JD, Cuthbert JA, Spady DK. Regulation of hepatic 7 alpha-hydroxylase expression by dietary psyllium in the hamster. J Clin Invest. 1994;93:2084–2092. doi: 10.1172/JCI117203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao L, Dawson PA, Woollett LA. Expansions of the bile acid pool size in pregnancy occurs even the absence of elevated pregnancy-induced sterol synthesis rates. Am J Physiol. 2003;284:G263–G268. doi: 10.1152/ajpgi.00332.2002. [DOI] [PubMed] [Google Scholar]
- 45.Reichen J, Karlaganis G, Kern F., Jr Cholesterol synthesis in the perfused liver of pregnant hamsters. J Lipid Res. 1987;28:1046–1052. [PubMed] [Google Scholar]
- 46.Wyne KL, Woollett LA. Transport of maternal LDL and HDL to the fetal membranes and placenta of the Golden Syrian hamster is mediated by receptor-dependent and receptor-independent processes. J Lipid Res. 1998;39:518–530. [PubMed] [Google Scholar]
- 47.Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Botolin D, Jump DB. Selective proteolytic processing of rat hepatic sterol regulatory element binding protein-1 (SREBP-1) and SREBP-2 during postnatal development. J Biol Chem. 2003;278:6959–6962. doi: 10.1074/jbc.M212846200. [DOI] [PubMed] [Google Scholar]
- 51.Bobard A, Hainault I, Ferré P, Foufelle F, Bossard P. Differential regulation of sterol regulatory element-binding protein 1c transcriptional activity by insulin and liver X receptor during liver development. J Biol Chem. 2005;280:199–206. doi: 10.1074/jbc.M406522200. [DOI] [PubMed] [Google Scholar]
- 52.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foufelle F, Ferré P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kennelly PJ, Rodwell VW. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by reversible phosphorylation-dephosphorylation. J Lipid Res. 1985;26:903–914. [PubMed] [Google Scholar]
- 55.Leoni S, Spagnuols S, Conti-Devirgiliis L, Dini L, Mangiantini MT, Trentalance A. Cholesterogenesis and related enzymes in isolated rat hepatocytes during pre- and postnatal life. J Cell Physiol. 1984;118:62–66. doi: 10.1002/jcp.1041180112. [DOI] [PubMed] [Google Scholar]
- 56.Kelley RI. Inborn errors of cholesterol biosynthesis. Adv Pediatr. 2000;47:1–52. [PubMed] [Google Scholar]
- 57.Tulenko TM, Boeze-Battaglia K, Mason RP, Tint GS, Steiner RD, Connor WE, Labelle EF. A membrane defect in the pathogenesis of the Smith-Lemli-Opitz syndrome. J Lipid Res. 2006;47:134–143. doi: 10.1194/jlr.M500306-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Shinitzky M, Borochov H, Wilbrandt W, Lassen UV, Ussing HH, Wieth JK. Membrane transport in erythrocytes. Alfred Benson Symposium 14; Munksgaard, Copenhagen: 1980. pp. 91–107. [Google Scholar]
- 59.Fielding CJ, Fielding PE. Membrane cholesterol and the regulation of signal transduction. Biochem Soc Trans. 2004;32:65–69. doi: 10.1042/bst0320065. [DOI] [PubMed] [Google Scholar]
- 60.Nikkila K, Miettinen TA. Serum cholesterol precursors, cholestanol, and plant sterols in primary biliary cirrhosis. Scand J Gastroenterol. 1988;23:967–972. doi: 10.3109/00365528809090155. [DOI] [PubMed] [Google Scholar]
- 61.Nikkila K, Hockerstedt K, Miettinen TA. High cholestanol and low campesterol-to-sitosterol ratio in serum of patients with primary biliary cirrhosis before liver transplantation. Hepatology. 1991;13:663–669. [PubMed] [Google Scholar]
- 62.Wasserman D, Zemel BS, Mulberg AE, John HA, Emerick KM, Barden EM, Piccoli DA, Stallings VA. Growth, nutritional status, body composition, and energy expenditure in prepubertal children with Alagille syndrome. J Pediatr. 1999;134:172–177. doi: 10.1016/s0022-3476(99)70411-7. [DOI] [PubMed] [Google Scholar]
- 63.Arvay JL, Zemel BS, Gallagher PR, Rovner AJ, Mulberg AE, Stallings VA, Haber BA. Body composition of children aged 1 to 12 years with biliary atresia or Alagille syndrome. J Pediatr Gastroenterol Nutr. 2005;40:146–150. doi: 10.1097/00005176-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Ramaccioni V, Soriano HE, Arumugam R, Klish WJ. Nutritional aspects of chronic liver disease and liver transplantation in children. J Pediatr Gastroenterol Nutr. 2000;30:361–367. doi: 10.1097/00005176-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Sokol RJ, Stall C. Anthropometric evaluation of children with chronic liver disease. Am J Clin Nutr. 1990;52:203–208. doi: 10.1093/ajcn/52.2.203. [DOI] [PubMed] [Google Scholar]


