Abstract
Experimental melanin-induced uveitis (EMIU) is a rodent model of acute anterior uveitis which was described in 1993. We investigated strain susceptibility, and age and gender characteristics of the model, undertook histological and immunohistochemical studies to investigate underlying cellular mechanisms, and examined several treatment options. Rats were immunized with bovine ocular melanin (250 μg), and disease was followed by slit lamp examination. Lewis, Fischer 344 and Porton rats were found to be susceptible to EMIU, whereas Wistar-Furth, DA, and Hooded Wistar strains were resistant. EMIU was neither age- nor gender-dependent. In Fischer 344 rats, EMIU was characterized clinically by florid anterior segment inflammation. Histopathological findings included infiltration of ciliary body and iris with mononuclear cells and neutrophils. Both CD4+ and CD8+ T lymphocytes were prominent. Rats were then treated with intraperitoneal injections of anti-CD4, anti-CD8 or irrelevant isotype-matched MoAb on days −3, 0, 3, 6 and 9 with respect to melanin immunization. Incidence of uveitis was significantly reduced in rats treated with a non-depleting cocktail of anti-CD4 MoAbs (P = 0.007), whereas a depleting anti-CD8 antibody had no effect on the disease. Mannose-6-phosphate inhibits lymphocyte migration in some models of T cell-mediated inflammation. This simple sugar was administered to additional rats via intraperitoneal osmotic pumps for 14 days following disease induction, but did not influence the uveitis. We conclude that EMIU is controlled by CD4+ T cells, and disease may be abrogated by treatment with anti-CD4 MoAbs.
Keywords: experimental melanin-induced uveitis, rat, T lymphocyte, anti-CD4 monoclonal antibody, mannose-6-phosphate
INTRODUCTION
Acute anterior uveitis (AAU) is a common inflammatory disorder of the iris and/or ciliary body, with an estimated lifetime cumulative incidence of 0.4% in the general population [1]. Individuals suffering from this condition experience a number of distressing symptoms including photophobia, ocular pain, decreased vision, lacrimation and vascular congestion. By definition, AAU resolves within 12 weeks [2], but 60% of patients suffer recurrent attacks of inflammation [3]. Recurrent disease ultimately results in permanent structural damage to the eye, which may cause blindness. Cataract, glaucoma, persisting posterior synechiae and cystoid macular oedema are all possible sequelae, and are found to occur in around one third of all patients [3]. Most cases of AAU are treated successfully with topical corticosteroids, although for severe attacks periocular or systemic corticosteroids may also be necessary. These drugs suppress inflammation non-specifically, and have serious side-effects even when locally administered. Adverse events include cataract, raised intra-ocular pressure which may eventually lead to glaucoma, and an increased susceptibility to microbial infection. There is also the risk of rebound inflammation on withdrawal of therapy.
The pathogenic processes operating in acute anterior uveitis have not yet been clarified, although the possibility of either immune complex- or cell-mediated autoimmune mechanisms has been debated [4]. Over 20 studies indicate that 19–88% of individuals with the condition carry HLA-B27, and AAU may coexist with other HLA-B27-linked diseases such as ankylosing spondylitis and Reiter's disease [5]. There is an unexplained association between Gram-negative bacterial infections and the HLA-B27-linked illnesses [6].
Elucidation of basic disease mechanisms is hampered by a lack of useful pathological specimens. Human tissues are generally only procured long after initiation of the inflammation, when complications have occurred. To date, endotoxin-induced uveitis (EIU) has been used almost exclusively as an animal model [7–9]. To induce uveitis, rats are injected systemically with lipopolysaccharide (LPS). Although the resultant inflammation involves the anterior uvea primarily, the time course is distinctly different from that of AAU. Clinical disease peaks at 24 h and resolves within a week. Furthermore, spontaneous recurrence does not occur. Experimental melanin-induced uveitis (EMIU) was first described in a series of reports published by Broekhuyse and co-workers in the early 1990s [10–12]. Following systemic immunization with bovine ocular melanin, Lewis rats develop an anterior uveal inflammation which mimics AAU in clinical appearance, duration and the occurrence of spontaneous relapse in a percentage of cases.
As EMIU has obvious clinical relevance, we wanted to characterize this model with regard to strain, sex and age susceptibilities. We examined the phenotypic characteristics of the inflammatory infiltrate in a relevant strain, and in the light of our findings attempted to prevent EMIU with MoAbs directed against T cell subsets. We also investigated the therapeutic potential of a simple sugar that has been shown to prevent ingress of lymphocytes into inflammatory sites.
MATERIALS AND METHODS
Animals
Male and female Lewis, Fischer 344, Wistar-Furth, DA, Porton and Hooded Wistar rats were used at age 10 weeks or less unless otherwise stated. Adult BALB/c mice were used for the production of MoAbs in ascites. Animals were housed at 21°C and 50% humidity in a 12-h light and 12-h dark cycle, and fed water and dried ration (New Joint Stock; Ridley Agriproducts, Murray Bridge, Australia). Experimental protocols were developed in accordance with the National Health and Medical Research Council (Australia) Statement for the Use of Animals in Research. All procedures and euthanasia were carried out under halothane (Fluothane; Zeneca Ltd, Macclesfield, UK) inhalation anaesthesia.
Induction and monitoring of experimental melanin-induced uveitis
Ocular melanin was extracted from bovine choroids according to the protocol described by Broekhuyse and colleagues [13], and quantified as a dry weight. In preliminary studies (unpublished data), Lewis rats were found to be susceptible to EMIU when immunized with bovine ocular melanin in doses ranging from 5 to 500 μg. However, a dose of 250 μg ensured the induction of maximally severe disease in all injected animals and was therefore chosen for the reported experiments. Rats received 125 μg of bovine ocular melanin in a 1:1 emulsion of sterile, non-pyrogenic normal saline and Hunter's TitreMax adjuvant (Sigma Chemical Co., St Louis, MO) by right hind footpad injection (60 μl). Immediately afterwards, they were injected intraperitoneally with the same quantity of melanin mixed with 1 μg of pertussis toxin (Sigma) in normal saline (40 μl). Animals were examined daily at the slit-lamp for clinical signs of uveitis, and findings were recorded using the clinical scoring system presented in Table 1. Studies were terminated at 28 days post-injection unless otherwise specified.
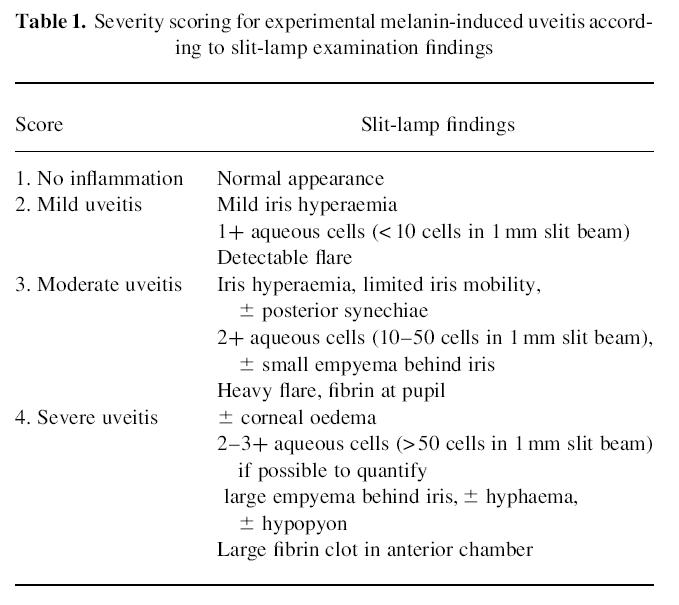
Table 1.
Severity scoring for experimental melanin-induced uveitis according to slit-lamp examination findings
Histological assessment of uveal inflammation
Enucleated eyes were fixed for a minimum of 24 h in 10% buffered formalin, dehydrated to 100% ethanol, further fixed for 18 h in chloroform, and embedded in paraffin wax. Tissue cross-sections were cut 5 μm thick and stained with haematoxylin and eosin. In order to draw histopathological correlations with clinical EMIU, groups of three Fischer 344 rats were killed at the time of injection and on days 1, 3, 5, 7, 10, 14 and 21, and weeks 6–8 of clinical disease. An additional group of eyes was collected immediately before onset of EMIU from animals with a delayed onset of disease in the second eye.
Monoclonal antibodies
Ms E. Hindmarsh and Dr D. Willenborg (Australian National University, Canberra, Australia) kindly provided murine ascites containing MoAb (IgG1 isotype) directed against the mannose-phosphate receptor (MPR). This was used at a dilution of 1:20 in Dulbecco's A PBS containing 1% v/v normal rat serum. Other mouse MoAbs used in the immunohistochemical identification of rat cell surface antigens were produced as undiluted supernatants from stationary phase hybridomas. Hybridomas X63 (unknown specificity; IgG1 isotype negative control), OX-1 (anti-CD45 (leucocyte-common antigen); IgG1 isotype), OX-35 and OX-38 (anti-CD4; IgG1 and IgG2a isotypes, respectively), OX-8 (anti-CD8; IgG1 isotype), NDS61 (anti-CD25 (IL-2 receptor (IL-2R)); IgG1 isotype), R73 (anti-T cell receptor (TCRαβ); IgG1 isotype), OX-26 (anti-CD71 (transferrin receptor); IgG2a isotype), OX-42 (anti-CD11b/CD18 (C3bi receptor); IgG2a isotype), ED-1 (anti-rat monocyte, macrophage and dendritic cell cytoplasmic antigen; IgG1 isotype) and OX-6 (anti-MHC monomorphic class II determinant; IgG1 isotype) were obtained from the European Collection of Animal Cell Cultures, and hybridoma SAL5 (anti-Salmonella antigen; IgG2a isotype-negative control) was a gift from Dr L. Ashman (University of Adelaide, Adelaide, Australia). To generate MoAb in ascites for the treatment trial, X63, SAL5, OX-35, OX-38 and OX-8 hybridoma cells were injected intraperitoneally into five separate groups of pristane-treated mice. The resultant ascites was diluted with sterile, pyrogen-free PBS containing 1% v/v fetal calf serum (FCS) to achieve an estimated antibody concentration of 0.2 mg/ml.
Immunohistochemical staining for cell surface antigens
Enucleated eyes from Fischer 344 rats were fixed in paraformaldehyde-lysine-periodate [14] for 4 h and dehydrated in 7% and 15% w/v sucrose in PBS. They were then embedded in Tissue-Tek OTC (Miles Inc., Elkhart, IN) and snap-frozen in liquid nitrogen. Ocular cross-sections were cut by cryostat at 8 μm thickness. Sections were incubated for 10 min at room temperature with 10% v/v normal swine serum (Commonwealth Serum Labs, Melbourne, Australia) in PBS, then with the primary antibody for 18 h. At this point and subsequently the sections were washed with PBS containing 0.2% w/v gelatin. They were incubated for 30 min with biotinylated affinity-isolated goat anti-mouse immunoglobulin (Dako Corp., Carpinteria, CA) diluted 1:500 in PBS containing 1% v/v normal rat serum, washed, and then incubated for a further 30 min with horseradish peroxidase (HRP)-conjugated streptavidin (Dako) diluted 1:1000 in PBS. Sections were washed and developed for 5 min in 9 mm Tris–HCl buffer at pH 7.6 with 40 mm sodium azide, 20 mm 3,3′-diaminobenzidine tetrahydrochloride, 9 mm imidazole, and 0.07% v/v hydrogen peroxide (Sigma). Finally they were rinsed in water and counter-stained with haematoxylin. To phenotype the inflammatory infiltrate, groups of three rats were killed at predetermined time points including days 1, 3, 5 and 7, and week 2 of clinical EMIU, and just before onset of disease. Eyes from three animals not immunized with melanin were also studied.
Treatment with anti-CD4 and anti-CD8 MoAbs
In a single experiment, Fischer 344 rats received five i.p. injections of mouse ascites, each containing approx. 200 μg of OX-8, or both OX-35 and OX-38 mixed 1:1, or X63 or SAL5 (1 ml). Injections were first given 3 days before disease induction, and were repeated on the day of melanin immunization and days 3, 6 and 9 thereafter. OX-35/OX-38-treated animals were followed for 56 days at the slit-lamp, whereas other animals were watched for 28 days. One rat from each treatment group was killed on day 3 of clinical EMIU, providing tissue for immunohistochemical examination. Twelve weeks after the first melanin injections all remaining OX-35/OX-38-treated animals, as well as three X63-treated and three SAL5-treated animals, were re-immunized according to the standard protocol.
During the treatment trial, peripheral T cell subsets were monitored by flow cytometry of tail blood preparations. Samples were collected from three rats in each of the four treatment groups just prior to the first antibody injection and on days 7, 14 and 28 after disease induction. Additional samples were taken from the OX-35/OX-38-treated animals 12 weeks after melanin immunizations. Up to 1 ml of peripheral blood was collected into a lithium heparin-coated tube, and diluted to 5 ml with HEPES-buffered RPMI 1640 medium (ICN Biomedicals Inc., Aurora, OH) containing 100 U/ml penicillin, 100 μg/ml streptomycin sulphate, 2 mm glutamine and 10% v/v FCS. Lymphocytes were separated using Lymphoprep (Nycomed Pharma As, Oslo, Norway) as described by the manufacturer, except that 40 mm meglumine diatrizoate (Angiografin; Schering AG, Berlin, Germany) was used to adjust the specific gravity of Lymphoprep to 1.09. The recovered cells were suspended in PBS–0.02 m sodium azide (PBS–azide) at 1 × 107 cells/ml, and to 50 μl of this lymphocyte suspension was added 50 μl of X63, SAL5, OX-35 or OX-8 MoAb. This mixture was incubated on ice for 30 min, then washed in PBS–azide and centrifuged at 400 g for 5 min at 4°C. The pellet was re-suspended in 25 μl of normal rat serum and 50 μl of FITC-conjugated goat anti-mouse immunoglobulin (Silenus Labs, Melbourne, Australia) diluted 1:50 with PBS–azide. A 30-min incubation on ice was followed by two consecutive washes. The cell pellet was re-suspended in 50 μl of fixative containing 10 mm glucose, 5% v/v formaldehyde and 5 mm sodium azide in PBS. Antibody binding was measured by flow cytometry using a standard fluorescein filter set (FACScan; Becton Dickinson, Mountain View, CA).
Treatment with mannose-6-phosphate
In a single experiment, Fischer 344 rats were treated with either mannose-6-phosphate or mannose control (Sigma). Both sugars were administered intraperitoneally via ALZET (Model 2ML2) osmotic pumps (ALZA Corp., Palo Alto, CA) which were primed and inserted in exact accordance with the manufacturer's instructions. Sugars were dissolved in sterile, non-pyrogenic PBS at a concentration of 40 mg/ml, and 4.7 μl were delivered each hour for 14 days, commencing 5 days after melanin immunizations. Rats were killed between days 3 and 7 of clinical EMIU, and one eye of each animal was examined histologically. All pumps were removed post mortem to verify full discharge of the contents.
Statistical analysis
Continuous variables (incidence of EMIU and incidence of severe (grade 4) EMIU) were analysed by the Mann–Whitney U-test, corrected for ties, and categorical data (day of onset of EMIU) were tested using the two-tailed Fisher's exact test.
RESULTS
Strain, sex and age susceptibility
Data presented in Table 2 describe the strain, sex and age susceptibility characteristics of EMIU. Lewis, Fischer 344 and Porton strains developed EMIU with no significant difference (P > 0.05) in incidence of disease. On the other hand, Wistar-Furth, DA and Hooded Wistar rats were resistant as judged by slit lamp examination, and further, showed no histological evidence of inflammation when both eyes were examined post mortem. Comparing susceptible rat strains, disease began significantly earlier in the Lewis than in the Fischer 344 (P < 0.001), which in turn began significantly earlier than in the Porton (P = 0.005). There was no significant difference in the incidence of clinically severe (grade 4) uveitis amongst these strains. Incidence, day of onset and clinical severity were identical for male and female Lewis or Fischer 344 rats. EMIU was also induced in a percentage of aged Fischer 344 rats, although uveitis was significantly delayed (P = 0.012), and the incidence of severe (grade 4) clinical uveitis was significantly lower (P < 0.001) than was observed amongst the younger animals.
Table 2.
Susceptibility to experimental melanin-induced uveitis (EMIU) according to rat strain, age and sex
Clinical course in the Fischer 344 rat and histopathological correlation
Earliest clinical signs of disease were small numbers of inflammatory cells and a protein flare in the aqueous humor, iris hyperaemia and a small or poorly reactive pupil. In most cases there was progression over the ensuing 24 h to a picture of florid inflammation which persisted for approximately 1 week before gradual resolution. The average duration of an attack was 24 days. In a group of nine animals followed for between 8 and 12 weeks post-immunization, five animals experienced relapsing inflammation.
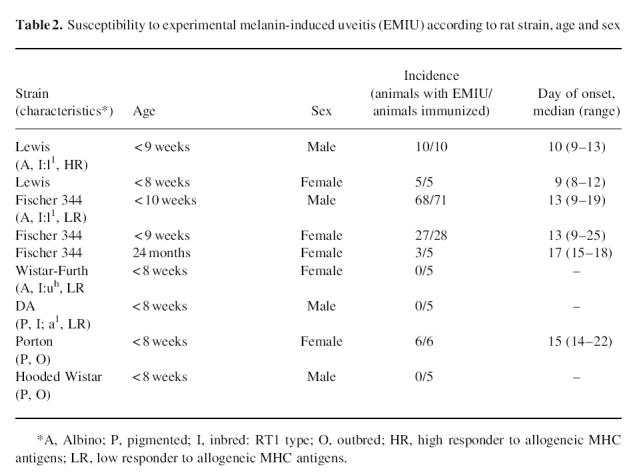
Immediately prior to the onset of clinical EMIU, leucocytes were scarce in the anterior uvea. However, as disease became clinically detectable, the basal ciliary body and iris were infiltrated with mononuclear cells and neutrophils. Changes had progressed by the third day, with swelling of the anterior uvea due to a largely mononuclear cell infiltrate, and an exudate in the anterior and posterior chambers dominated by neutrophils (Fig. 1a). Inflammation remained severe until the middle of the second week, although neutrophil numbers were markedly reduced from 3 days onwards. In the most severe cases limbitis and vitritis were observed (Fig. 1b,c), as well as choroiditis. By 3 weeks after the onset, there was minimal uveal inflammation. However, the iris appeared abnormal with loose stroma and disturbance of the epithelial layers. Iris architecture remained disturbed a further month later. Histological inflammation at this later time point was always in the context of relapsing clinical inflammation.
Fig. 1.
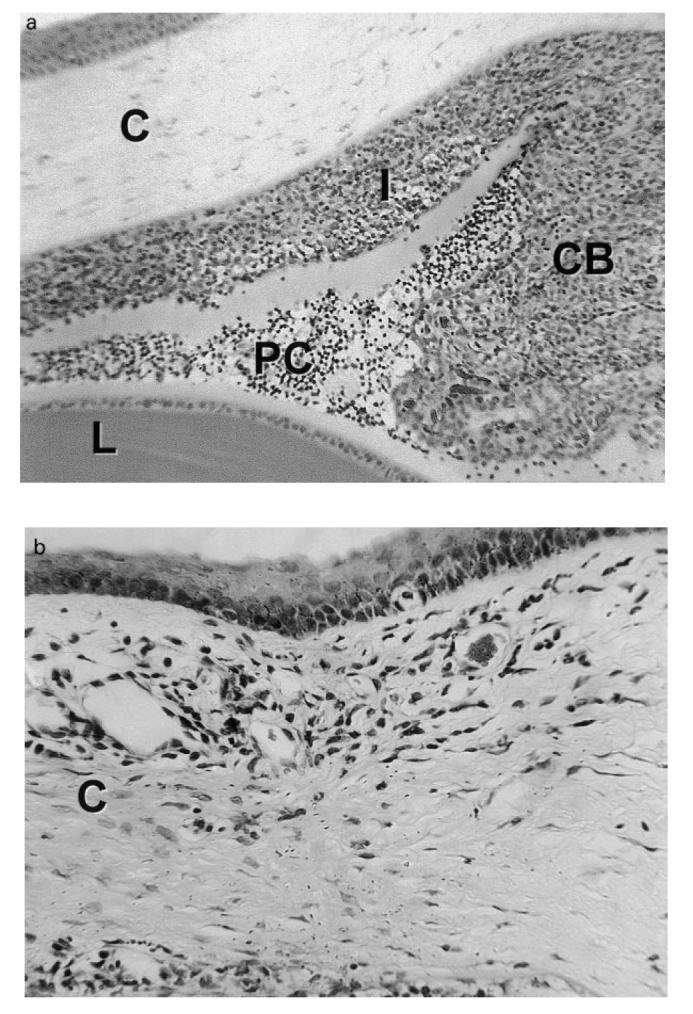
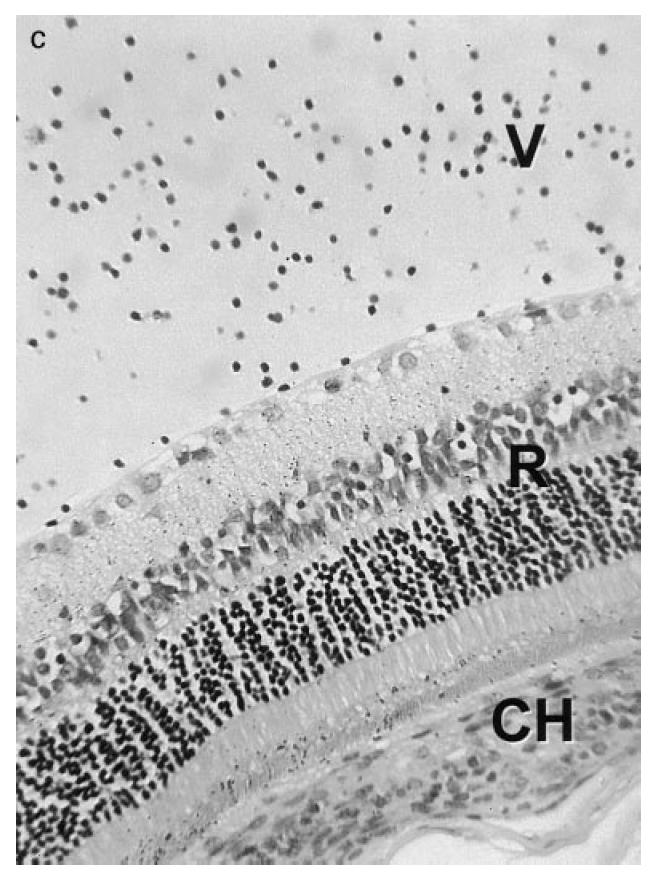
(see next page.) Photomicrographs of the eye of a Fischer 344 rat during the first week of experimental melanin-induced uveitis showing (a) massive swelling of the ciliary body (CB) and iris (I) due to infiltration by mononuclear cells and neutrophils with spill-over into the posterior chamber (PC); (b) an inflammatory infiltrate in the peripheral cornea (C) or limbus, and (c) inflammatory cells within the vitreous (V). L, Lens; CH, choroid; R, retina. Stain: haematoxylin and eosin. (Original mag.: (a) ×1000; (b) ×2500; and (c) ×1500.)
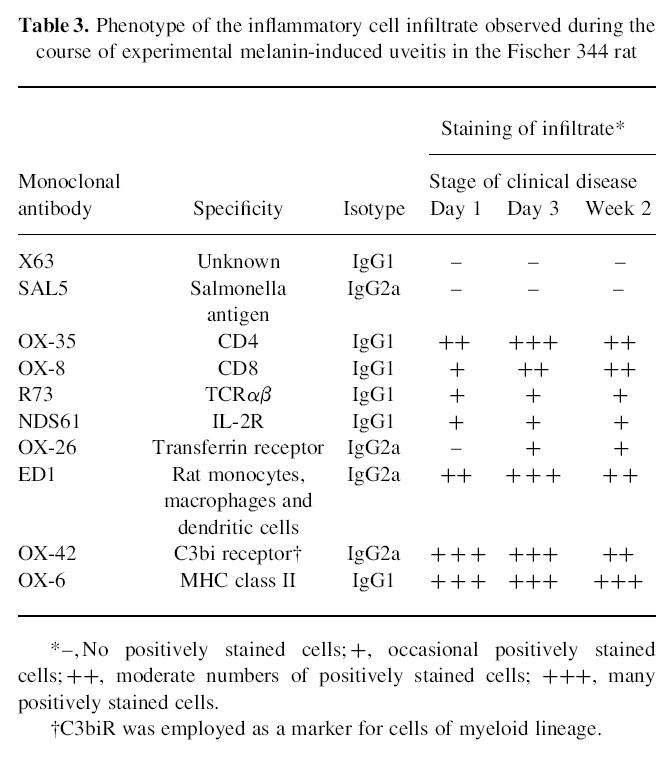
Phenotype of the inflammatory cell infiltrate
Expression of selected cell surface markers is presented in Table 3. Times tabulated are the day of onset of EMIU, day 3 when the eye was maximally inflamed and week 2 when resolution of the disease had begun. At the onset of clinical EMIU, the cells infiltrating the eye included many CD4+ T lymphocytes, as well as macrophages and neutrophils. Small numbers of CD8+ T cells were also present from the outset, but by day 7 this T cell complement was equal to or exceeded CD4+ numbers. IL-2R and TCRαβ were detectable on the first day of disease, and CD71 became detectable by day 3. Up-regulation of MHC class II antigen was seen throughout the uveal tract and on the cornea prior to the onset of EMIU. Many infiltrating cells stained positive for MPR which was undetectable in the normal rat uvea.
Table 3.
Phenotype of the inflammatory cell infiltrate observed during the course of experimental melanin-induced uveitis in the Fischer 344 rat
Anti-CD4/anti-CD8 MoAb treatment trial
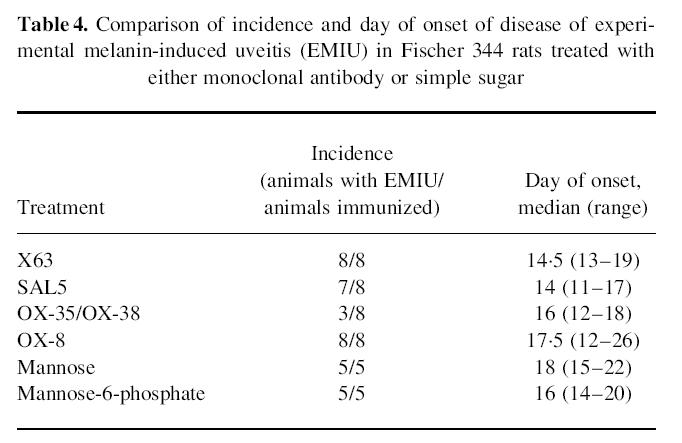
Flow cytometry demonstrated a modest peripheral depletion of CD4+ T lymphocytes, but complete peripheral depletion of CD8+ cells after OX-35/OX-38 and OX-8 treatments, respectively (Fig. 2). The incidence of EMIU was significantly reduced (P = 0.007) for the group of rats treated with OX-35/OX-38 when compared with control animals receiving either X63 or SAL5. However, there was no significant difference between incidence or time of onset for OX-8-treated rats and X63-treated controls (Table 4). Clinical severity was similar amongst groups. The phenotypic composition of the uveal infiltrate was identical in the four eyes representing each treatment. In particular, CD4+ T cells were present in the eye of an OX-35/OX-38-treated rat that developed disease. Twelve weeks after the initial melanin injections, remaining animals in control and anti-CD4-treated groups were re-immunized with melanin. There was near-complete recovery of the peripheral CD4+ T cell population in the latter group at this time. Whereas disease appeared as early as day 4 postimmunization in control animals, four of seven OX-35/OX-38-treated animals did not develop EMIU, including three of the five that had not previously developed disease.
Fig. 2.
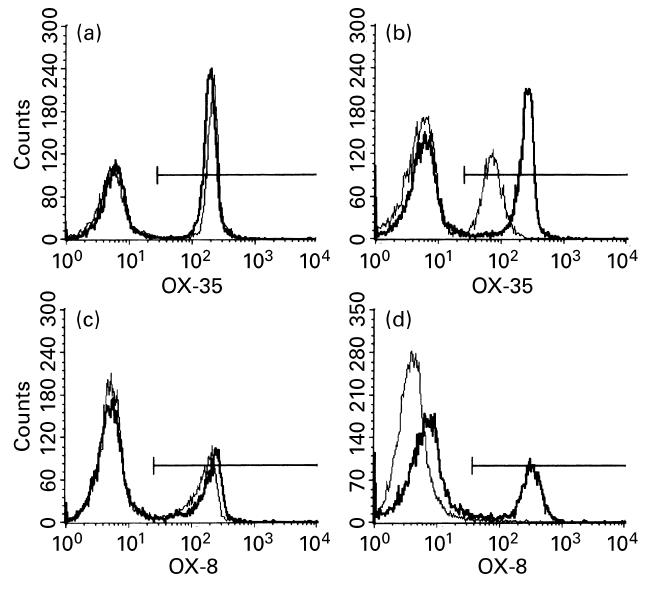
Flow cytometry histograms illustrating a modest peripheral depletion of CD4+ T lymphocytes (from 50% to 35% of positive cells) for one representative rat treated with OX-35/OX-38 ((a) baseline and (b) 7 days following disease induction), and complete peripheral depletion of CD8+ T lymphocytes (from 30% to 0% positive cells) for a second rat treated with OX-8 ((c) baseline and (d) 7 days following disease induction). Electronic gating selected lymphocytes from other peripheral blood cells for the purposes of the analysis. Bold lines represent an X63-treated control rat, and narrow lines represent the OX-35/OX-38-treated or OX-8-treated rat. The gates used to select for positive cells are also shown.
Table 4.
Comparison of incidence and day of onset of disease of experimental melanin-induced uveitis (EMIU) in Fischer 344 rats treated with either monoclonal antibody or simple sugar
Mannose-6-phosphate treatment trial
Clinical EMIU was not significantly different with respect to total incidence or day of onset for rats receiving mannose-6-phosphate when compared with those given mannose (Table 4). Furthermore, disease was equally severe in both groups according to clinical examination. Lymphocyte numbers were not reduced in the infiltrate observed in the mannose-6-phosphate-treated group.
DISCUSSION
Experimental melanin-induced uveitis was first described in Lewis rats [10–12]. We found that Fischer 344 (inbred albino) and Porton (outbred albino) rats were also susceptible to induction of this disease model, but that Wistar-Furth (inbred albino), DA (inbred pigmented) and Hooded Wistar (outbred pigmented) rats were resistant. Lewis and Fischer 344 are RT1 haplo-identical, but the finding that EMIU could be induced in the outbred Porton rat indicates that MHC haplotype cannot be the sole determinant of disease susceptibility. In high-responder strains, including the Lewis and Wistar-Furth, CD8+ T cells can be activated by allogeneic MHC antigens independently of CD4+ T cells. In contrast, CD8+ T cells from low-responder strains such as PVG and DA require help from CD4+ T cells for effective activation [15]. In common with most humans [15], the Fischer 344 is a low-responder strain (Professor B. M. Hall, personal communication). Plainly, susceptibility to EMIU is independent of alloantigen responder status. However, the Fischer 344 rat appears to be a particularly suitable strain for studies of the pathogenesis and treatment of acute anterior uveitis, given that its immune response closely approximates the human.
Human AAU may be recurrent, most commonly occurs in adults and is equally severe in males and females. In the Fischer 344 rat, EMIU lasted approximately 1 month and recurred spontaneously in half of those animals followed long term. We were able to induce EMIU in rats aged 2 years, as well as young animals, and found no gender-related differences in severity. In contrast, EIU has been reported to be more severe in male Lewis rats than in females [16]. Susceptibility to EIU is also age-dependent. Lewis rats aged ≤ 8 weeks are susceptible to disease whereas those > 10 weeks are resistant [17]. The recurrent nature, and age and gender independence of EMIU increase its applicability as an animal model of human AAU.
The histopathological picture of EMIU in Fischer 334 rats was similar to that previously described in the Lewis strain [10,18,19], but with some differences. Heavy infiltration of the ciliary body and iris with mononuclear cells and polymorphonuclear leucocytes was observed, with spill-over into the anterior and posterior chambers and an associated choroiditis. Additional features in the Fischer 344 rat included limbitis and vitritis. The significance of the choroidal involvement in EMIU is a controversial issue. Occurrence of choroiditis during EMIU has led some to regard this disease model as one resembling recurrent panuveitis in humans [18]. On the other hand, Broekhuyse et al. [20] and others [19,21] maintain that this is a useful model of acute anterior uveitis. We first observed inflammation in the anterior uvea, and this tissue was also the most severely inflamed throughout the attack. Interestingly, posterior segment findings including choroiditis, vitritis, vitreous haemorrhage, retinal vasculitis, retinal haemorrhage and inflammatory cell infiltration of the retina with focal destruction of photoreceptor cells have been observed during rat EIU [22,23]. Obviously, in the absence of histopathological studies from human eyes, it is not possible to exclude absolutely choroidal involvement in some cases of human acute anterior uveitis.
Previous immunohistochemical studies in the Lewis rat [18,23,24] have documented a heterogeneous inflammatory infiltrate including T lymphocytes, macrophages and neutrophils, although reported proportions and time of appearance of the different leucocyte subpopulations varied. In the Fischer 344 rat, CD4+ cells were present in large numbers early in the course of disease. CD8+ cells were detected from the outset, albeit in low numbers. As resolution of EMIU began, their numbers rose to equal that of the CD4+ subpopulation. Activation markers including IL-2R, TCRαβ and the transferrin receptor were detected early, suggestive of an antigen-driven response. Macrophages and granulocytes were also present, their numbers rising and falling in parallel with the course of the inflammation.
The histological and immunohistochemical findings suggest that EMIU is a cell-mediated antigen-specific disease, with features most in keeping with a DTH immune response. Ear swelling responses and lymphocyte proliferation studies [18], as well as successful adoptive transfer of uveitis by CD4+ T cells [11] in immunized Lewis rats, all support this conclusion. Therapies directed against the T cell or against molecules controlling cell migration into the eye seem the most logical options for the treatment of EMIU.
The incidence of EMIU was significantly reduced in rats treated systemically with a combination of OX-35 and OX-38 MoAbs, directed against two different epitopes of rat CD4. The treatment induced only a slight peripheral depletion of CD4+ cells. Furthermore, when the OX-35/OX-38-treated animals were re-immunized with melanin, three of five remained disease-free. In contrast, there was no significant difference in incidence, time of onset or disease severity in rats treated with OX-8, a MoAb directed against rat CD8, despite a complete peripheral depletion of CD8+ cells in these animals. The findings suggest that EMIU is controlled by CD4+ T cells, and that a short course of immunosuppression using anti-CD4 MoAb may be sufficient to control disease in the long term. Lifelong maintenance of transplantation tolerance has been achieved in adult mice by short courses of anti-CD4 and anti-CD8 antibodies [25].
Two mannose-6-phosphate receptors function primarily to transport acid hydrolases produced in the Golgi apparatus to lysosomes. In addition, when present on the cell surface these receptors may anchor acid hydrolases and allow these enzymes to degrade proteoglycans of the extracellular matrix [26]. Leucocytes may use this mechanism to degrade basement membrane and migrate into inflammatory sites. Mannose-6-phosphate has been shown to interfere specifically with lymphocyte migration, presumably by displacing acid hydrolase from its receptor [27,28]. Treatment with mannose-6-phosphate inhibits other models of cell-mediated autoimmune disease in rodents, including adjuvant arthritis [29], experimental autoimmune encephalomyelitis [30] and thyroid allograft rejection [31].
Unexpectedly, when MPR was targeted using mannose-6-phosphate in a dosing regimen comparable to that used successfully to inhibit the other models, incidence, time of onset and severity of EMIU were not influenced. Furthermore, histological examination revealed a typical, predominantly lymphocytic tissue infiltrate. Infiltrating leucocytes expressed MPR, but apparently did not require this for movement into the eye. This result implies that a specific feature of the anterior uveal vessels allows T cells to enter the eye by unusual mechanisms. Perhaps relevant is the observation that ciliary body contains fenestrated capillaries with pores that may occupy the full circumference of the vessel [32]. In this tissue, the blood–ocular barrier is located at the level of the non-pigmented epithelium. An undefined peculiarity of the ciliary body vasculature has also been blamed for the unique susceptibility of the anterior uvea to endotoxin-induced inflammation [33].
In conclusion, experimental melanin-induced uveitis is a cell-mediated, antigen-driven autoimmune anterior uveitis which has clinical features in common with human AAU, and complements EIU in the investigation of pathogenic mechanisms operating in this disease. The florid and recurrent nature of the inflammation is ideal for the assessment of potential therapies. We have found that the CD4+ T lymphocyte controls EMIU, and that treatment targeting this cell may be useful in the management of uveitis.
Acknowledgments
The authors thank Michelle Lewis and Loretta Wheatland for technical assistance, Raymond Yates for animal husbandry and Angela Chappell for slit lamp photography. This work was supported in part by grants from the National Health and Medical Research Council of Australia, the Ophthalmic Research Institute of Australia and the Flinders Medical Centre Research Foundation.
REFERENCES
- 1.Linssen A, Rothova A, Valkenberg HA, Dekker-Saeys AJ, Luyendijk L, Kijlstra A, Feltkamp TEW. The lifetime cumulative incidence of acute anterior uveitis in a normal population and its relation to ankylosing spondylitis and histocompatibility antigen HLA-B27. Invest Ophthalmol Vis Sci. 1991;32:2568–78. [PubMed] [Google Scholar]
- 2.Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–5. doi: 10.1016/s0002-9394(14)74235-7. [DOI] [PubMed] [Google Scholar]
- 3.Rothova A, Van Veenendaal WG, Linssen A, Glasius E, Kijlstra A, De Jong PTVM. Clinical features of acute anterior uveitis. Am J Ophthalmol. 1987;103:137–45. doi: 10.1016/s0002-9394(14)74218-7. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan HJ. Immunologic insights into the eye and uveitis. In: Kraus-Mackiw E, O'Connor GR, editors. Uveitis pathophysiology and therapy. Stuttgart: Thieme; 1986. pp. 227–44. [Google Scholar]
- 5.Wakefield D, Montanaro A, McCluskey P. Acute anterior uveitis and HLA-B27. Surv Ophthalmol. 1991;36:223–32. doi: 10.1016/0039-6257(91)90005-z. [DOI] [PubMed] [Google Scholar]
- 6.Feltkamp TEW, Muhammad AK, Lopez de Castro JA. The pathogenetic role of HLA-B27. Immunol Today. 1996;17:5–7. doi: 10.1016/0167-5699(96)80559-7. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–3. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacherjee P, Williams RN, Eakins KE. An evaluation of ocular inflammation following the injection of bacterial endotoxin into the rat foot pad. Invest Ophthalmol Vis Sci. 1983;24:196–202. [PubMed] [Google Scholar]
- 9.Cousins SW, Guss RB, Howes EL, Rosenbaum JT. Endotoxin-induced uveitis in the rat: observations on altered vascular permeability, clinical findings, and histology. Exp Eye Res. 1984;39:665–76. doi: 10.1016/0014-4835(84)90065-4. [DOI] [PubMed] [Google Scholar]
- 10.Broekhuyse RM, Kuhlmann ED, Winkens HJ, Van Vugt AHM. Experimental autoimmune anterior uveitis (EAAU), a new form of experimental uveitis. I. Induction by a detergent-insoluble, intrinsic protein fraction of the retinal pigment epithelium. Exp Eye Res. 1991;52:465–74. doi: 10.1016/0014-4835(91)90044-f. [DOI] [PubMed] [Google Scholar]
- 11.Broekhuyse RM, Kuhlmann ED, Winkens HJ. Experimental autoimmune anterior uveitis (EAAU). II. Dose-dependent induction and adoptive transfer using a melanin-bound antigen of the retinal pigment epithelium. Exp Eye Res. 1992;55:401–11. doi: 10.1016/0014-4835(92)90112-6. [DOI] [PubMed] [Google Scholar]
- 12.Broekhuyse RM, Kuhlmann ED, Winkens HJ. Experimental autoimmune anterior uveitis (EAAU). III. Induction by immunisation with purified uveal and skin melanins. Exp Eye Res. 1993;56:575–83. doi: 10.1006/exer.1993.1071. [DOI] [PubMed] [Google Scholar]
- 13.Broekhuyse RM, Kuhlmann ED. Experimental autoimmune anterior uveitis. The preparation of uveitogenic ocular melanin. Invest Ophthalmol Vis Sci. 1993;34:698–700. [PubMed] [Google Scholar]
- 14.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–83. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 15.Hall BM. Cells mediating allograft rejection. Transplantation. 1991;51:1141–51. doi: 10.1097/00007890-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Baer JC, Morris SM. Anterior chamber inflammation in endotoxin induced uveitis is greater in male Lewis rats than in females. ARVO Abstracts. Invest Ophthalmol Vis Sci. 1993;34:1475. [Google Scholar]
- 17.Hoekzema R, Verhagen C, Van Haren M, Kijlstra A. Endotoxin-induced uveitis in the rat. The significance of intraocular interleukin-6. Invest Ophthalmol Vis Sci. 1992;33:532–9. [PubMed] [Google Scholar]
- 18.Chan C-C, Hikita N, Dastgheib K, Whitcup SM, Gery I, Nussenblatt RB. Experimental melanin-protein-induced uveitis in the Lewis rat. Immunopathologic processes. Ophthalmology. 1994;101:1275–80. doi: 10.1016/s0161-6420(94)31199-7. [DOI] [PubMed] [Google Scholar]
- 19.Bora NS, Kim MC, Kabeer NH, Simpson SC, Tandhasetti MT, Cirrito TP, Kaplan AD, Kaplan HJ. Experimental autoimmune anterior uveitis. Induction with melanin-associated antigen from the iris and ciliary body. Invest Ophthalmol Vis Sci. 1995;36:1056–66. [PubMed] [Google Scholar]
- 20.Broekhuyse RM, Winkens HJ, Kuhlmann ED. Multiple recurrences in melanin-protein-induced uveitis in the rat. Ocul Immunol Inflamm. 1995;3:149–55. doi: 10.3109/09273949509069107. [DOI] [PubMed] [Google Scholar]
- 21.Okumura A, Mochizuki M. Endotoxin-induced uveitis in rats: morphological and biochemical study. Jpn J Ophthalmol. 1988;32:457–65. [PubMed] [Google Scholar]
- 22.Ruiz-Moreno JM, Thillaye B, De Kozak Y. Retino-choroidal changes in endotoxin-induced uveitis in the rat. Ophthalmic Res. 1992;24:162–8. doi: 10.1159/000267163. [DOI] [PubMed] [Google Scholar]
- 23.Kim MC, Kabeer NH, Tandhasetti MT, Kaplan HJ, Bora NS. Immunohistochemical studies on melanin associated antigen (MAA) induced experimental autoimmune anterior uveitis (EAAU) Curr Eye Res. 1995;14:703–10. doi: 10.3109/02713689508998498. [DOI] [PubMed] [Google Scholar]
- 24.McMenamin PG, Crewe J, Kijlstra A. Resident and infiltrating cells in the rat iris during the early stages of experimental melanin protein-induced uveitis (EMIU) Ocul Immunol Inflamm. 1997;5:223–33. doi: 10.3109/09273949709085063. [DOI] [PubMed] [Google Scholar]
- 25.Qin S, Cobbold S, Benjamin R, Waldmann H. Induction of classical transplantation tolerance in the adult. J Exp Med. 1989;169:779–94. doi: 10.1084/jem.169.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornfeld S. Structure and function of the mannose 6-phosphate/insulin-like growth factor II receptors. Annu Rev Biochem. 1992;61:307–30. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett MR, Cowden WB, Parish CR. Differential effects of the anti-inflammatory compounds heparin, mannose-6-phosphate, and castanospermine on degradation of the vascular basement by leucocytes, endothelial cells, and platelets. J Leukoc Biol. 1995;57:207–13. doi: 10.1002/jlb.57.2.207. [DOI] [PubMed] [Google Scholar]
- 28.Parish CR, Hindmarsh EJ, Bartlett MR, Staykova MA, Cowden WB, Willenborg DO. Treatment of central nervous system inflammation with inhibitors of basement membrane degradation. Immunol Cell Biol. 1998;76:104–13. doi: 10.1046/j.1440-1711.1998.00722.x. [DOI] [PubMed] [Google Scholar]
- 29.Willenborg DO, Parish CR, Cowden WB. Inhibition of adjuvant arthritis in the rat by phosphosugars and the α-glucosidase inhibitor castanospermine. Immunol Cell Biol. 1992;70:369–77. doi: 10.1038/icb.1992.49. [DOI] [PubMed] [Google Scholar]
- 30.Willenborg DO, Parish CR, Cowden WB. Phosphosugars are potent inhibitors of central nervous system inflammation. FASEB J. 1989;3:1968–71. doi: 10.1096/fasebj.3.8.2721857. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett MRE, Warren HS, Cowden WB, Parish CR. Effects of the anti-inflammatory compounds castanospermine, mannose-6-phosphate and fucoidan on allograft rejection and elicited peritoneal exudates. Immunol Cell Biol. 1994;72:367–74. doi: 10.1038/icb.1994.55. [DOI] [PubMed] [Google Scholar]
- 32.Raviola G. The structural basis of the blood–ocular barriers. Exp Eye Res. 1977;25(S):27–63. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- 33.Herbort CP, Chan CC, Nussenblatt RB. Endotoxin-induced uveitis in the rat: a hypothesis for preferential involvement of the anterior uvea. Curr Eye Res. 1990;9(S):119–24. doi: 10.3109/02713689008999430. [DOI] [PubMed] [Google Scholar]


