Abstract
CD4+ and CD8+ T cells from healthy donors, acute rheumatic fever (ARF) and chronic rheumatic heart disease (CRHD) patients responded variably to a superantigen from Streptococcus pyogenes—Streptococcal pyrogenic erythrogenic toxin A (SPE-A). In vitro culture of CD4+ T cells from ARF patients (CD4-ARF) with SPE-A exhibited a Th1 type of response as they produced high levels of IL-2, while CD4+ T cells from CRHD patients (CD4-RHD) secreted IL-4 and IL-10 in large amounts, i.e. Th2 type of cytokine profile. The skewing of human CD4+ T cells (in response to SPE-A stimulation) to Th1 or Th2 type reflects the role of the two subsets in a disorder with differing intensities at the two extremes of the spectrum. Moreover, the anergy induction experiments revealed that CD8-ARF and CD8-RHD undergo anergy (to different extents), whereas CD4+ T cells do not, in response to re-stimulation by SPE-A. These results initially demonstrate that both CD4+ and CD8+ T cells respond differentially to SPE-A, and hence it is an important observation with respect to the pathogenesis of ARF/CRHD. Anergy in CD8+ T cells in the presence of SPE-A in vitro goes a step further to show the clinical relevance of these cells and their possible role in suppression of the disease.
Keywords: superantigen, CD4+/CD8+ cells, acute rheumatic fever, chronic rheumatic heart disease
INTRODUCTION
Streptococcus pyogenes, or group A streptococcus, is a common pathogen of the pharynx and skin. This at times can lead to late, non-suppurative sequelae such as rheumatic fever, glomerulonephritis, and other illnesses such as scarlet fever, etc. [1, 2]. Bacterial superantigens have been implicated in the pathogenesis of infectious diseases [3, 4]. These superantigens are found as pathogenic bacterial exotoxins. Streptococcal exotoxins (erythrogenic toxins) are potent stimulators of T cells [5]. The toxin under study is Streptococcal pyrogenic erythrogenic toxin A (SPE-A), and it stimulates T cells in the presence of antigen-presenting cells (APC) whose MHC is necessary for binding [6]. SPE-A shares its biological activities with other members of the pyrogenic toxin family. These activities include pyrogenicity, enhancement of lethal endotoxin shock and superantigenicity [7]. The gene for SPE-A has been identified in 32–80% of the streptococcal isolates, suggesting a potential role of this exotoxin in pathogenesis [8]. T cells play an important role in most of the infectious diseases where CD4+ T cells regulate the acquired cellular immune response providing protection and CD8+ T cells work in co-ordination to bring about the required effect [9]. CD4+ lymphocytes have Th1 and Th2, the subsets which secrete a signature lymphokine profile, i.e. Th1 secrete IL-2 and interferon-gamma (IFN-γ); Th2 secrete IL-4, IL-5, IL-6 and IL-10 [10]. CD8+ T cells also have subsets which exhibit Th1-like (Tc1) and Th2-like (Tc2) profile [11].
Since the role of SPE-A in the pathogenesis of acute rheumatic fever (ARF)/chronic rheumatic heart disease (CRHD) has not been very well defined, we undertook a study of the responses of T cells from normal donors, ARF patients and CRHD patients. For this study, we performed two types of experiments. In the first, we directly examined the in vitro stimulation of CD4+ and CD8+ T cells from different groups by the superantigen SPE-A and their differentiation based on the cytokine profile. In the second, we carried out anergy induction by SPE-A to detect responsiveness or unresponsiveness in T cell subsets.
PATIENTS AND METHODS
Patient population and isolation of peripheral blood mononuclear cells
Known patients with ARF and CRHD attending the Cardiology Clinic at the graduate Institute of Medical Education and Research (Chandigarh, India) were enrolled, based on the clinical findings, anti-streptolysin O (ASO) and C-reactive protein (CRP) status and absence of any other infections. The diagnosis of ARF was based on updated Jones' criteria [12]. CRHD was assumed when the patient had manifestation of involvement of the mitral valve on two-dimensional echocardiography, with or without a previous history of rheumatic fever [13]. A total of 12 patients with ARF and 10 with CRHD were included in the study. The mean age of the ARF patient was 23.4 ± 6 years (range 13–35 years), whereas the mean age of the CRHD patients was 38 ± 5 years (range 22–54 years). The normal donors enrolled in the study totalled 14, aged 19–36 years. After obtaining informed consent, 15–20 ml of whole blood containing 10 U/ml heparin were obtained, diluted in an equal volume of PBS and centrifuged over Ficoll–Hypaque density gradient (Sigma Chemical Co., St Louis, MO) for 20 min at 150 g at 20°C. The cells from the interface were washed and then cultured in RPMI 1640 (Difco Labs, Detroit, MI) supplemented with antibiotics (70 mg penicillin and 110 mg streptomycin per litre medium), 2 mml-glutamine, 2 mm sodium pyruvate, 10 mm HEPES, 5 × 10−5m 2-mercaptoethanol and 10% AB+ serum (complete culture medium).
Isolation of CD4+ and CD8+ T cells
The T cells positive for surface expression of CD4+ or CD8+ were purified on a nylon wool column [14]. The non-adherent cell population (mainly T cells) was collected and washed. To avoid B cell contamination, the cells were incubated in plates coated with goat anti-human immunoglobulin (10 mg/ml). The negatively selected cells were incubated with anti-CD4+ (OKT4) and panned a second time on culture flasks coated with anti-CD8+ (OKT8). Both the antibodies were purified from culture supernatant on a protein-A column and were used at 10 mg/ml for coating. These separations were monitored by staining with fluoresceinated antibodies on FACScan (Becton Dickinson, Mountain View, CA). This protocol yielded > 98% pure T cells with CD4+ or CD8+ markers. All cells were re-suspended in complete culture medium at a final concentration of 1 × 106 cells/ml.
APC
The adherent population was eluted out from the 75-cm2 culture flasks with 0.05 m EDTA/PBS and treated with mitomycin C [14]. Briefly, cells at a concentration of 1–6 × 107 cells/ml were treated with 25 μg of mitomycin C (Sigma) per ml of cell suspension at 37°C for 20 min in the dark. The cells were washed extensively with culture medium containing 5% fetal calf serum (FCS).
T cell proliferation
CD4+ or CD8+ T cells (2 × 104 cells/well) were co-cultured with mitomycin C-treated adherent cell population (5 × 105 cells/well) in a 96-well flat-bottomed plate (Nunc, Roskilde, Denmark) in a humidified incubator in an atmosphere of 5% CO2 at 37°C. As positive control, anti-CD3 (20 ng/ml) was added to the cultures. The SPE-A, supplied by Dr W. Kohler (Institut für Experimentelle Mikrobiologie, Jena, Germany), was tested for the presence of endotoxin by the limulus amoebocyte lysate assay [15]. Also, as a precautionary measure the SPE-A preparation was passed through a polymyxin B resin before use. All solutions used for the experiments and the SPE-A preparation itself were demonstrated to be lipopolysaccharide (LPS)-free (< 0.0025 ng/ml) before use. During the last 16 h of this 72-h culture, 1 μCi/well tritiated thymidine was added. The cells were later harvested by a PHD harvester, and the filter discs were dried and added to scintillation vials containing 5 ml non-aqueous scintillation fluid. The amount of DNA synthesized was measured by the level of 3H-thymidine incorporation. Specific activity of 3H-thymidine used was 18 000 mCi/mmol.
Cytokine assays
The supernatant of a 24-h culture in the same conditions as for proliferation was collected and used for ELISA. All assays were carried out in triplicates. The standards used permitted the determination in the supernatant of a minimum concentration of 150 pg/ml of IL-4, 110 pg/ml of IL-2 and 195 pg/ml of IL-10 (Genzyme, Cambridge, MA). The antibodies used were goat anti-human IL-2, IL-4 and IL-10 and horseradish peroxidase (HRP)-conjugated anti-goat (Dako, Glostrup, Denmark). HRP activity was revealed by OPD substrate (Sigma). Plates were read at 490 nm on a microplate ELISA reader (Molecular Devices, Sunnyvale, CA]. IFN-γ levels were measured using detection kit (Endogen, Woburn, MA) according to the manufacturer's instructions. It could detect < 2 pg/ml of human IFN-γ.
Induction of T cell anergy
T cells (106 cells/ml) from all three groups under study were incubated for 16 h with 0.5 μg/ml SPE-A. Controls such as (i) T cells in medium only, (ii) T cells with SPE-A and anti-SPE-A, (iii) T cells with immobilized anti-CD3 antibody (12–20 μg/ml) and T cells with recombinant human IL-2 (Genzyme) were performed in parallel, i.e. incubated for 16 h. At the end of incubation, the cells were collected on Ficoll by density gradient centrifugation, washed with RPMI 1640 and rested for 5 days. These washed cells were then incubated with SPE-A and (i) autologous mitomycin C-treated APC, (ii) autologous mitomycin C-treated APC and recombinant IL-2.
Statistical analysis
All measurements are expressed as mean ± s.d. Unpaired t-test was used to assess the results. Comparison between various results obtained during the course of experimentation were made, i.e. ARF and CRHD were compared with normals and P < 0.05 was taken as significant.
RESULTS
Dose–response curve
Human CD4+ T cells or CD8+ T cells enriched from PBMC of healthy donors, ARF and CRHD patients were stimulated with SPE-A in the presence of mitomycin C-treated adherent cell population. SPE-A was titrated on CD4+ and CD8+ T cells to obtain a dose–response curve. A suboptimal response was found at 50 ng/ml for CD4+ and CD8+ T cells (Fig. 1). As shown in Fig. 1, the CD4+ population was a better responder to SPE-A than CD8+ T cells. Anti-CD3 was the most potent stimulus, augmenting proliferation several-fold.
Fig 1.
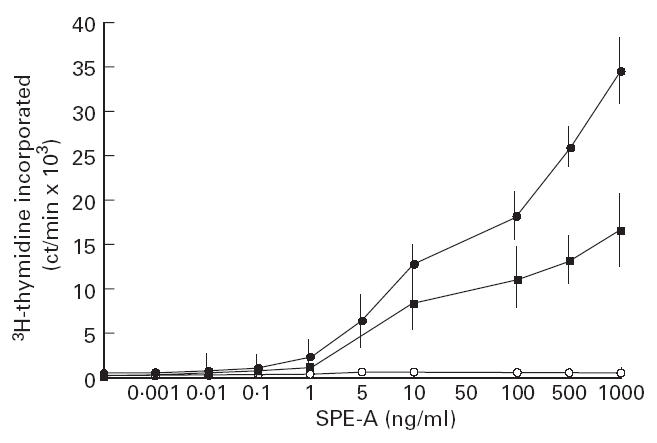
Dose–response curve. Proliferative response of CD4+ and CD8+ cells upon in vitro stimulation with Streptococcal pyrogenic erythrogenic toxin A (SPE-A). CD4+ or CD8+ T cells (2 × 104) and 105 adherent, mitomycin C-treated autologous population from healthy donors, were co-cultured with varying amounts of SPE-A (•, CD4; ▪, CD8), anti-CD3 (20 ng/ml) alone (ct/min 86 462 ± 5710), anti-CD3 (20 ng/ml) with anti-SPE-A (80 926 ± 6515), anti SPE-A (6 μg/ml) raised in rabbit (○), normal rabbit serum (1:500 dilution). Normal rabbit serum was unable to cause inhibition of SPE-A (data not shown). The cultures were pulsed with 3H-thymidine after 48 h, for 16 h and data are presented as mean ct/min ± s.d., where experiments were conducted in triplicates.
In order to rule out the role of any contaminating agents in stimulation of T cells by SPE-A, we used a specific polyclonal antibody against SPE-A (raised in rabbit) at 6 μg/ml and a control normal rabbit serum at 1:500 dilution. The antiserum to SPE-A completely neutralized the response to 10 ng/ml SPE-A at 6 μg/ml. The response to anti-CD3 was not affected by this serum.
Responsiveness/non-responsiveness
Finally we investigated the role of SPE-A in induction of anergy in CD4+/CD8+ T cells from normal, ARF and CRHD individuals. The cells were initially incubated with SPE-A for 16–20 h in the absence of APC to check for induction of unresponsiveness, so that they were unable to proliferate in response to an immunogenic challenge with SPE-A in the presence of APC. We then compared anergy induction upon exposure to SPE-A in the three above mentioned groups. The state of anergy was reversed on addition of exogenous IL-2. CD4+ T cells from all three groups exhibited very mild unresponsiveness, if any, upon re-stimulation with the antigen (Fig. 2). They proliferated, although to a lesser extent, in response to the superantigenic challenge after a 5-day rest in the absence of any exogenous cytokine.
Fig 2.
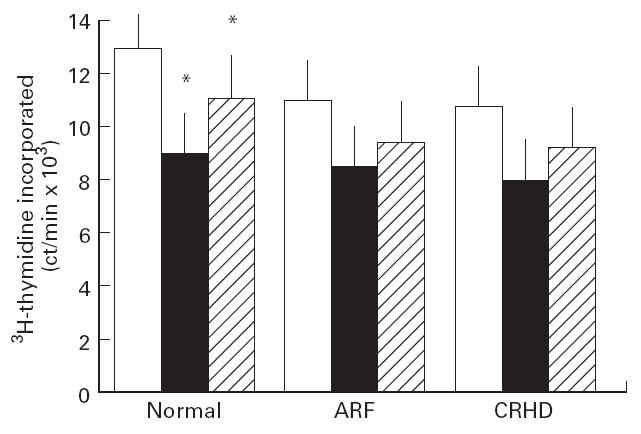
Effect of repeated dose of Streptococcal pyrogenic erythrogenic toxin A (SPE-A) on CD4+ T cells. CD4+ T cells (105 cells/ml) from all the three groups under study were incubated initially with 50 ng/ml SPE-A for 16–20 h in the absence of any antigen presenting cells (APC) in a 24-well tissue culture plate. The cells were collected on Ficoll, washed and rested for 5 days. After rest, the cells were restimulated with SPE-A in the presence of autologous mitomycin C-treated APC and cell proliferation was measured by 3H-thymidine uptake (abscissa). Recombinant IL-2 along with APC and SPE-A was added to check for reversal of anergy. Controls: T cells alone (ct/min 437 ± 64), T cells + SPE-A (542 ± 72), T cells incubated with SPE-A and anti-SPE-A followed by addition of T cells + SPE-A + APC (ct/min 26 542 ± 2472) and normal cells + anti-CD3 (74 829 ± 3641), acute rheumatic fever (ARF)-CD4 T cells + anti-CD3 (92 461 ± 5212), chronic rheumatic heart disease (CRHD)-CD8 T cells + anti-CD3 (93 298 ± 4718) were included. Addition of anti-SPE-A to these anti-CD3 control wells did not bring any significant change in proliferation data. All the controls were treated in parallel to the test samples under similar conditions. Results are depicted as (ordinate): open blocks, stimulation with SPE-A in the presence of APC as done in Fig. 1; black blocks, restimulation as done for anergy induction; hatched blocks, response to exogenous IL-2. *P < 0.05, response in presence and absence of IL-2 in normal CD4+ T cells.
CD4+ T cells from normal donors had a better proliferative response to SPE-A in the presence of APC (P < 0.05) than their ARF or CRHD counterparts. The restimulation done for anergy induction was slightly different in ARF and CRHD compared with the normals, but not significantly.
Results from anergy experiments conducted with CD8+ T cells were different. Although CD8+ T cells from normal individuals did not undergo unresponsiveness, those from ARF and CRHD showed marked unresponsiveness (P < 0.01). CD8+ T cells from the ARF and CRHD patients were different, as there was a weak response to the agonist on re-stimulation in the presence of APC (Fig. 3). The unresponsiveness of CD8+ T cells to SPE-A in the presence of APC was not due to cell death as the cells were viable (> 85% cells viable). They showed reasonable proliferation on addition of exogenous IL-2 (P < 0.05), although to variable levels in ARF and CRHD (Fig. 3), suggesting that the unresponsiveness observed was the result of anergy induced in cells. Comparison of the post-anergy ARF-CD8+ T cell induction in presence and absence of exogenous IL-2 showed a significant difference (P < 0.01) between the two conditions. At the same time, post-anergy induction of CRHD-CD8+ T cells in presence or absence of exogenous IL-2 indicated a very conspicuous difference (P < 0.005). In all these experiments, T cells alone or T cells with SPE-A as controls did not proliferate, indicating absence of APC as contaminants in T cell preparation.
Fig 3.
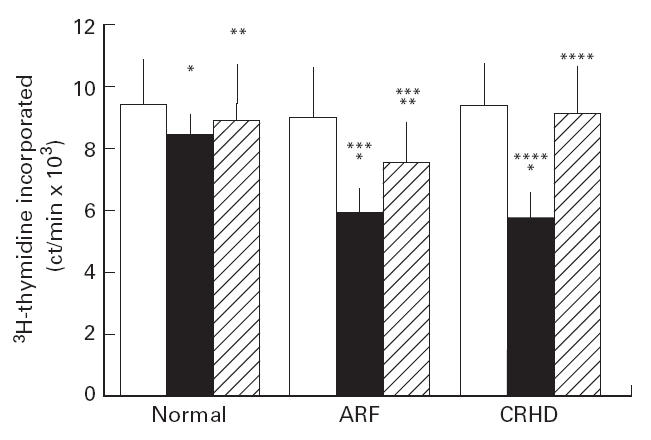
Induction of anergy in CD8+ T cells. CD8+ T cells (105 cells/ml) from all three groups were cultured with Streptococcal pyrogenic erythrogenic toxin A (SPE-A; 50 ng/ml) in the absence of antigen-presenting cells (APC) for 16–20 h. These cells were collected on Ficoll, washed and rested for 5 days. Restimulation with SPE-A autologous mitomycin C-treated APC was measured by the amount of 3H-thymidine incorporated by proliferating cells (abscissa). Reversal of anergy was brought about by addition of APC, SPE-A and exogenous IL-2. T cells alone (ct/min 368 ± 52]; T cells + SPE-A (625 ± 48); T cells incubated with SPE-A and anti-SPE-A followed by T + SPE-A + APC (ct/min 204 22 ± 4214) and normal T cells + anti-CD3 (80 325 ± 3552); acute rheumatic fever (ARF)-CD8 T cells + anti-CD3 (84 293 ± 4881); chronic rheumatic heart disease (CRHD)-CD8 T cells + anti-CD3 (82 969 ± 5238). Addition of anti-SPE-A to the anti-CD3 control wells did not bring about any change in the proliferation data. Open blocks: stimulation with SPE-A ipo APC as done in Fig. 1; black blocks, restimulation as done for anergy induction; hatched blocks, response to exogenous IL-2. *P < 0.05, restimulation in ARF and CRHD compared with normal; **P < 0.01, response to exogenous IL-2 in normal and ARF; ***P < 0.01, comparison of response in absence and presence of IL-2 in ARF; ****P < 0.005, comparison of response in absence and presence of IL-2 in CRHD.
Cytokine production by SPE-A-induced T cells
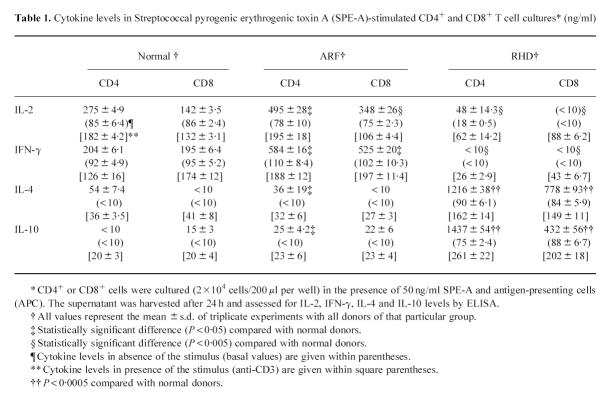
The SPE-A-induced CD4+ cells produced variable amounts of cytokines and this helped in determining the subtype predominantly existing in ARF and CRHD compared with healthy control cells. As shown in Table 1, activation of CD4-ARF with SPE-A caused an increased IL-2 and IFN-γ production, although their IL-4 and IL-10 production was markedly decreased compared with CD4-CRHD. The lowered IL-2 and IFN-γ production and the augmented IL-4 and IL-10 production were observed in the CD4-CRHD cells. Normal CD4+ T cells produced large amounts of IL-2 and IFN-γ and much lower quantities of IL-4 and IL-10. The basal levels of all samples, i.e. CD4+ or CD8+ T cells without SPE-A, were low. Anti-CD3 was able to bring about regular interleukin secretion, emphasizing that the variations observed in the presence of SPE-A are specific to it. It appears that CD4-ARF are endowed with Th1-type function. whereas CD4-CRHD cells exhibit a Th2-like cytokine pattern. This kind of Th1–Th2 dichotomy remains an important functional division in the immune system.
Table 1.
Cytokine levels in Streptococcal pyrogenic erythrogenic toxin A (SPE-A)-stimulated CD4+ and CD8+ T cell cultures* (ng/ml)
Variations in CD8+ T cells
The CD8+ T cells obtained from the different patients and healthy donors were also co-cultured with SPE-A and APC for induction. The cytokines so generated were detected by ELISA (Table 1). We show that CD8-ARF released high levels of IL-2 and IFN-γ, while CD8-RHD secreted minimal levels of these cytokines. Interestingly, CD8-RHD produced IL-4 in moderate amounts, while none of it was detected in CD8-ARF. IL-10 was detectable in CD8-ARF but not to the extent seen in CD8-CRHD, i.e. approx. 20-fold higher. Levels of interleukins in the presence of anti-CD3 showed that stimulation could induce certain levels which were different from the basal levels (activated cellular state). These results demonstrate that dichotomy in CD8+ T cells also existed in ARF/CRHD. A subset of CD8+ T cells in ARF secreted Th1-like cytokine profile, Tc1, while the subset in CD8-RHD resembled the Th2 pattern, i.e. Tc2.
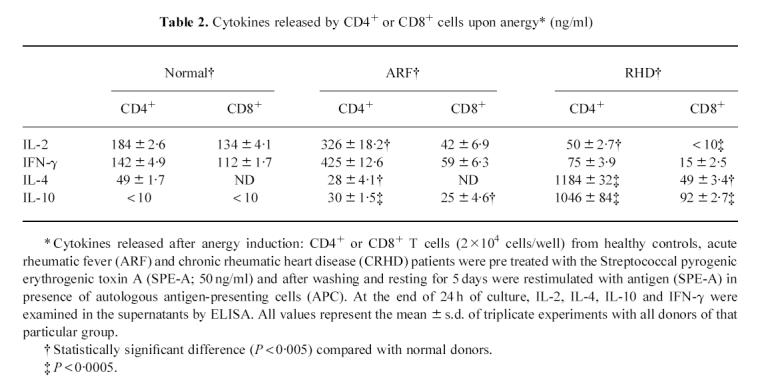
The cytokines released by CD4+ and CD8+ cells under anergic conditions also demonstrated the activity of the cells. The CD4+ T cells secreted IL-2, IFN-γ, IL-4 and IL-10 nearly in the same pattern as they did during regular stimulation without pretreatment with antigen alone (Table 2). This and the proliferation data suggest that CD4+ T cells are basically unaffected by antigenic pretreatment and hence do not undergo anergy. The CD8+ T cells from the ARF and CRHD group exhibited decreased levels of interleukin secretion. This was obvious when restimulation of cells pretreated with SPE-A resulted in decreased levels of IL-2 and IFN-γ in CD8-ARF and IL-4 as well as IL-10 levels in CD8-CRHD. This is an indication of anergy induction in CD8 T cells from patients.
Table 2.
Cytokines released by CD4+ or CD8+ cells upon anergy* (ng/ml)
 |
DISCUSSION
A lot of interest has recently been generated in research on bacterial superantigens, focusing on their mechanism of T cell activation and their pathogenic role in infection or autoimmune disease [4]. The bacterial superantigens are basically exotoxins which might have implications in the pathogenesis of infections together with related diseases, which at times may manifest acute or chronic clinical symptoms [16]. The present study was conducted to discover clues regarding pathogenesis in the non-suppurative sequelae of Streptococcal infection, i.e. ARF and CRHD. The cytokine levels of SPE-A-activated CD4+ T cells showed increasing levels of IL-2 from CD4+ T cells as CRHD < normal < ARF. IL-4 and IL-10 secretion by CD4+ T cells revealed low levels in normal donors, moderate levels in ARF cases and reasonably high levels in CRHD patients. Our study clearly indicates that under acute conditions, CD8+ T cells have a strong bias towards a Tc1 subtype, while a Tc2-like cytokine profile is seen in CRHD. This kind of distinct division of signature cytokines in ARF and CRHD suggests that a Th1–Th2 decision is being taken at some point, hence a Th1-type of pattern in ARF and a Th2 kind of profile in RHD CD4+ T cells was observed upon superantigenic stimulation. Also, subsets of CD8+ T cells have been identified in humans, as well as in mice during infections [11, 17], where Tc1 cells produce IL-2 and IFN-γ whereas a Tc2-like pattern is IL-4, IL-5 and IL-10.
A role of (i) antigen dose and (ii) cytokines [18] can not be excluded. As reported by Hosken et al. [19], antigen dose is an important factor in Th1–Th2 decision. Weigle [20] is of the view that the amount of antigen presented to T cells has an important bearing on both tolerance and immunity. It is possible that high concentrations of the antigen lead to repeated T cell stimulation, thus increasing IL-4 production and Th2 development, or induce a state of immunological tolerance, which often preferentially shuts off Th1 cells [21, 22]. Too little antigen can selectively shift the balance to a Th1-type response [23]. Cytokines themselves can be strong stimuli for influencing cytokine patterns, as quantitative differences in cytokine expression may lead to stimulation of variable conditions. For example, IL-2 is known to enhance synthesis of IFN-γ [18]. Also, IL-4, a Th2 promoting regulator, would increase the frequency of cells producing at least one Th2 cytokine. In earlier studies, Schmitz et al. [24] have shown that T cells themselves produce small amounts of IL-4 from their initial activation and the amount of IL-4 that accumulates at the site of T cell response increases with increasing lymphocyte activation. The Th2-inducing effect of IL-4 dominates over the other cytokines [25], so that if IL-4 levels reach a necessary threshold, Th2 differentiation is initiated and IL-4 production increases progressively. This explains increasingly pronounced Th2 responses with repeated T cell stimulation. The role of IL-4-induced CD8+ Th2 cells, i.e. Tc2, is also known to have great relevance to immune responses against infectious agents. IL-4 rapidly induces Tc1 clones to lose their ability to synthesize cytokines, particularly IL-2 [26].
The cytokine signals induced by the pathogen can induce appropriate types of T cells and effector functions. Keeping these in mind, it can be hypothesized that in ARF, the Th1 polarity can be attributed to their stimulation by moderate levels of antigens. CRHD, being a valvular sequelae, is a case of chronic set up with ongoing immune reactions. We observed a Th2-type of response in this case, which could suggest that the sensitized lymphocytes of these patients were responding to a wider range of antigenic challenge.
The cytokines released by CD4+ and CD8+ cells under anergic conditions also speak for the cells. The CD4+ T cells secreted the IL-2, IFN-γ, IL-4 and IL-10 nearly in the same pattern as they did during regular stimulation without a pretreatment with antigen alone (Table 2). This and the proliferation data suggest that CD4+ T cells are basically unaffected by antigenic pretreatment and hence do not undergo anergy. The CD8+ T cells from the ARF and RHD group exhibited decreased levels of interleukin secretion. This was obvious when restimulation of cells pretreated with SPE-A resulted in decreased levels of IL-2 and IFN-γ in CD8-ARF and IL-4 as well as IL-10 levels in CD8-RHD.
The anergy experiment shows that exposure to SPE-A leads to reduced activation, which could be a strategy of balancing immunity and tolerance by turning down the activation of these superantigens and self-reactive cells. Thus, the T cells (in the present study CD8 cells) have become tolerant and are not easily activated into clonal expression after presentation of superantigen [27].
Probably the division of Th1 (Tc1) phenotype in ARF and a protective Th2 (Tc2) phenotype suggest a mechanism for induction of peripheral self tolerance/anergy in the T cell population. In the absence of APC initially, SPE-A, which otherwise brought about immense proliferation, made the CD8+ T cells unresponsive to a subsequent antigenic challenge in the presence of APC. This resembled the unresponsiveness of T cells induced by free antigen in peptidic form [28] or that presented by chemically modified accessory cells [29]. ARF and CRHD being post-streptococcal sequelae are a case of molecular mimicry [30], and to our surprise CD4+ T cells did not exhibit unresponsiveness as did CD8+ T cells. Anergy in CD4+ T cells has been reported previously [31], but lack of anergy in CD4+ T cells only reflects a conditional state. This implies a condition where TCR–SPE-A–MHC complex is bound stably (high affinity) and activation is triggered. Also, restimulation of post-anergy CD4+ T cells in absence and presence of IL-2 is not very different in ARF and CRHD. CD8+ T cells have been reported by Yan et al. [32] to be anergic in response to bacterial superantigens in immune cases. CD8+ T cells in our study became anergic, but were not inert, as they might have participated in immune responses under different circumstances. On comparing the post-anergy induction of these cells in presence and absence of IL-2, we found a significant difference in ARF and CRHD cases.
Earlier studies by Yan et al. [32] showed that in vitro stimulation and restimulation of murine splenic T cells with Staphylococcal enterotoxin E induced memory CD4+ T cells. These cells have increased reactivity in proliferation and lymphokine production in response to restimulation with Staphylococcal enterotoxin E. CD8+ T cells under similar conditions exhibit non-reactivity, i.e. they seem to be rendered tolerant. So far, there is not enough available data to explain why the fate of CD4+ and CD8+ T cells is so distinctly different. The in vitro stimulation of isolated human CD4+ and CD8+ T cells with SPE-A resulted in expansion of both of them, although the patterns of reactivity were different in the two subsets (as has been discussed earlier). In the light of the above findings and our study, it seems likely that the superantigen-induced abnormal reactions were T cell-dependent where CD4+ T cells exhibited stimulating activity and secreted lymphokines which were able to increase the reactions. As a defensive mechanism, CD8+ T cells were rendered tolerant. This superantigen-induced tolerance can be the mechanism for minimizing the superantigen-induced abnormal reactions as well as for escaping harmful attacks by autoreactive lymphocytes.
The finding of toxin-induced anergy demonstrates that an extrinsic superantigen can functionally inactivate the response of human T cells, which may affect their functional capacity. This anergy could be due to complex molecular regulation, and therefore knowledge of anergy induction by a superantigen could be of help in understanding the pathophysiological mechanism in ARF or CRHD [33]. Two major arguments find support in our study: (i) T helpers have different roles to play in ARF and CRHD; (ii) unresponsiveness of CD8+ cells throws light on a possible point of therapeutic intervention, as CD8+ T cells as well as Th2 cells can act as regulatory cells for cellular immune responses.
In conclusion, we have shown that SPE-A-reactive CD8+ T cells which appear anergic in vitro are functional cells with peculiar behaviour. They have an arrested proliferation which reflects an unrecognized physiological consequence of in vivo triggering (as ARF/CRHD patients are pre-exposed to antigen). It is not known how long this anergy is retained in vivo, and if so, are they then terminally differentiated cells?
Acknowledgments
The authors express their acknowledgement to Sudhir Kumar for secretarial assistance.
References
- 1.Cone LA, Woodard DR, Schlievert PM, Tomory GS. Clinical and bacteriology observations of a toxic shock like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317:146–9. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972;126:294–301. doi: 10.1093/infdis/126.3.294. [DOI] [PubMed] [Google Scholar]
- 3.Kotb M. Role of superantigens in infectious diseases and their sequelae. Curr Opin Infect Dis. 1992;5:364–74. [Google Scholar]
- 4.Drake CG, Kotzin BL. Superantigens: biology, immunology and potential role in disease. J Clin Immunol. 1992;12:149–62. doi: 10.1007/BF00918083. [DOI] [PubMed] [Google Scholar]
- 5.Janeway CJ, Yagi J, Caurad PJ, Katz ME, Jones B, Vroegop S, Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behaviour. Immunol Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson R, Fischer H, Sjogren HO. Binding of Staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T-cells. J Immunol. 1988;140:2484–8. [PubMed] [Google Scholar]
- 7.Bohach GA, Fast O, Nelson RD, Schlievert PM. Staphylococcal and Streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–72. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 8.Bronze MS, Dale JB. The re-emergence of serious group A streptococcal infections and acute rheumatic fever. Am J Med Sci. 1996;311:41–54. doi: 10.1097/00000441-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Quaratino S, Murison G, Knyba RE, Verhoef A, Londei M. Human CD4+ and CD8+ oc β T-cells express a functional T-cell receptor and can be activated by superantigens. J Immunol. 1991;147:3319–23. [PubMed] [Google Scholar]
- 10.Mossmann TR, Cherwinski H, Bond MW, Gidlin MA, Coffman RL. Two types of murine helper T-cell clones I. Definition according to profiles of lymphokines, activities and secreted proteins. J Immunol. 1986;136:2348–52. [PubMed] [Google Scholar]
- 11.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. Differing lymphokine profiles of functional subsets of human CD4+ and CD8+ T-cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Prevention of Rheumatic Fever, Endocarditis, Kawasaki Disease and Cardiovascular Disease in Young. American Heart Association, Jones' Criteria 1992 update. JAMA. 1992;268:2069–73. [PubMed] [Google Scholar]
- 13.Nichol PM, Gilbert BW, Kisslo JA. Two dimensional echocardiographic assessment of mitral stenosis. Circulation. 1977;55:20–128. doi: 10.1161/01.cir.55.1.120. [DOI] [PubMed] [Google Scholar]
- 14.Mishell BB, Shiigi SM. Selected methods in cellular immunology. New York: Freeman WH & Co.; 1980. pp. 182–5. [Google Scholar]
- 15.E-Toxate [limulus amebocyte lysate] for detection and semi-quantitation of endotoxin. St Louis: Sigma; 1995. Tech Bulletin [210] [Google Scholar]
- 16.Kotzin B, Leung DYM, Kappler S, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 17.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarised antigen specific CD8 effector populations: reciprocal action of interleukin-4 and IL-12 in promoting type-2 and type-1 cytokine profiles. J Exp Med. 1994;180:1715–28. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mossman TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 19.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T-helper cell phenotype development in a T-cell receptor β-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weigle WO. Analysis of autoimmunity through experimental models of thyroiditis and allergic encephalomyelitis. Adv Immunol. 1980;30:159–273. doi: 10.1016/s0065-2776(08)60196-0. [DOI] [PubMed] [Google Scholar]
- 21.DeWit D, Mechelen MV, Ryelandt M, Figueiredo AC, Abramowicz D, Goldman M, Bazin H, Leo O. The injection of deaggregated gamma globulins in adult mice induces antigen specific unresponsiveness of T helper type 1 but not type 2 lymphocytes. J Exp Med. 1992;175:9–15. doi: 10.1084/jem.175.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burstein HJ, Abbas AK. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993;177:457–63. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parish CR, Liew FY. Immune response to chemically modified flagellin. J Exp Med. 1972;135:298–11. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz J, Andreas T, Kuhn R, Rajewsky K, Muller W, Assenmacher M, Radbruch A. Induction of interleukin 4 (IL-4) expression in T helper (Th) cells is not dependent on IL-4 from non-Th cells. J Exp Med. 1994;179:1349–53. doi: 10.1084/jem.179.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1, CD4+ T cells through IL-12 produced by Listeria induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 26.Sad S, Mosmann TR. Interleukin 4, in the absence of antigen stimulation, induces an anergy like state in differentiated CD8+ TC1 cells: loss of IL-2 synthesis and autonomous proliferation but potential of cytotoxicity and synthesis. J Exp Med. 1995;182:1505–15. doi: 10.1084/jem.182.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rammensee H-G, Kroschweski R, Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunising Mls1b mice with Mls1a expressing cells. Nature. 1989;339:541–4. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- 28.Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldmann M. Induction of tolerance in influenza virus immune T lymphocyte clones with synthetic peptides of influenza hemagglutinins. J Exp Med. 1983;157:1434–47. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T-cell antigen receptor occupancy. Ann Rev Immunol. 1989;7:445–80. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 30.Kuby J. Autoimmunity. In: Kuby J, editor. Immunology. 2. New York: W.H. Freeman; 1994. pp. 445–66. [Google Scholar]
- 31.Gaus H, Miethke T, Wagner H, Heeg K. Superantigen-induced anergy of Vb8+, CD4+ T cells induces functional but non-proliferative T cells in vivo. Immunol. 1994;83:333–40. [PMC free article] [PubMed] [Google Scholar]
- 32.Yan X-J, Li X-Y, Imanishi K, Kumazawa Y, Uchiyama T. Study of activation of murine T-cells with bacterial superantigens. In vivo induction of enhanced responses in CD4+ T-cells and anergy in CD8+ T-cells. J Immunol. 1993;150:3873–81. [PubMed] [Google Scholar]
- 33.Oldstone MBA. Molecular mimicry and autoimmune disease. Cell. 1987;50:819–20. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]