Abstract
A family with three cases of macroglobulinaemia of undetermined significance (MGUS), and one case each of immunoblastic lymphoma, Waldentröm's macroglobulinaemia and multiple myeloma was first described 20 years ago. We have previously identified 10 out of 35 healthy family members tested whose lymphocytes produced abnormally high amounts of immunoglobulins in culture. In the present study lymphocyte subpopulations of these hyper-responders have been further characterized and lymphocyte reactivity and survival in vitro have been studied. No differences were detected in the proportions of resting B lymphocytes (CD19+) co-expressing CD5, CD10, CD11b, or CD38, and the CD4/CD8 ratio of T cells was normal before and after stimulation with pokeweed mitogen (PWM). The initial rate of response in terms of immunoglobulin production was not increased, but immunoglobulin levels continued to rise during the second week of culture whereas the production peaked at 8 days in control cultures. This was associated with significantly greater survival of lymphocytes and at 14 days surviving B cells could only be identified in samples from hyper-responders. A lymph node removed because of tuberculosis from a family member 23 years before the diagnosis of multiple myeloma showed very marked Bcl-2 expression in a B cell follicle. This was not seen in a tuberculous lymph node from an unrelated subject. Stimulated cultures from three hyper-responders tested demonstrated significantly higher retention of Bcl-2 in B cells compared with one family control and six unrelated controls. We conclude that the increased production of immunoglobulins previously observed in this family with an inherited tendency for benign and malignant B cell proliferation is the result of enhanced B cell survival, which is associated with increased expression of Bcl-2 following stimulation.
Keywords: macroglobulinaemia, MGUS, Bcl-2, B cell survival
INTRODUCTION
The importance of programmed cell death (apoptosis) in the development of mature B and T cells as well as in the regulation of immune responses is now well recognized [1,2]. Death signals are delivered to cells by signalling cascades, triggered through surface receptors such as Fas [3]. The sensitivity of a cell to these death signals depends on Bcl-2 and related molecules, such as Bax, Bcl-XL and Bcl-XS. These molecules form homo- or heterodimers in the mitochondrial membrane and some of these can form membrane channels. The composition of these dimers can determine the initiation of the later stages of programmed cell death. Thus apoptosis is prevented by a preponderance of dimers containing Bcl-2, whereas Bax promotes the activation of cell death proteases [4,5].
Inappropriate cell survival in the immune system may result in benign and malignant lymphoproliferative disorders and autoimmunity. Thus mutations in the Fas gene are manifested as lymphoproliferative syndromes, sometimes with additional autoimmune symptoms, in mice and humans [3]. The Bcl-2 gene was described originally as the gene involved in the t14:18 translocation characteristically associated with follicular B cell lymphomas. Transgenic mice with hyperactive Bcl-2 develop lymphomas and may also express autoimmunity, dependent on genetic background [6,7]. No hereditary disorders in man have so far been ascribed to the Bcl-2 gene.
An Icelandic family with macroglobulinaemia and B cell malignancies was first described in 1978 [8]. At that time one case of immunoblastic lymphoma was diagnosed, and two cases of benign monoclonal gammopathy along with one case of Waldenström's macroglobulinaemia were identified in the same sibship. The family was investigated for evidence of macroglobulinaemia and nine asymptomatic family members were found to have polyclonally elevated serum levels of IgM. In one of these family members multiple myeloma was diagnosed 9 years later. Around 25 similar families have been reported in the literature [9,10]. Some of these families have also shown signs of autoimmunity, but there is no clinical or laboratory evidence of autoimmunity in the Icelandic family. In 1989 blood samples were again collected from 35 family members and tested for in vitro responses to mitogens. No differences were detected in proliferative responses but samples from 10 family members showed increased production of IgG, IgA and IgM, defined as > 3 s.d. above the mean for a group of unrelated control subjects. These 10 family members will be referred to as hyper-responders. Their position in the pedigree suggested heredity [10]. For the present study further samples were collected from family members in 1991 and 1994, with the aim of analysing further the possible mechanisms behind this hyper-responsiveness of B cells. To this end we analysed B and T cell subpopulations, measured cell survival and studied Bcl-2 expression in resting cells and following stimulation.
SUBJECTS AND METHODS
Subjects and samples
Peripheral blood samples were collected, using EDTA as anticoagulant, from nine family members on two different occasions; six of these were previously known to show abnormally high production of immunoglobulins in vitro and were thus classified as hyper-responders (H), three family members had been classified as normal responders (N). On each occasion samples were collected from the same number of healthy control donors (C) of the same age and sex. Mononuclear cells were prepared by centrifugation through Ficoll–Hypaque (Histopaque; Sigma, St Louis, MO). Part of each sample was used fresh for measurements, as detailed below, the remainder was cryopreserved for later use. For the study of phenotypic markers and Bcl-2 expression cryopreserved samples were used, including those from the first sample collection from 35 family members as well as control samples from the Icelandic Cancer Society's biological specimen bank. Sections of paraffin-embedded tissue samples from patients belonging to the family and selected control patients were obtained from the Dungal collection of archival tissue, Department of Pathology, University of Iceland, Reykjavik, Iceland.
Cell culture
Culture was performed in 2-ml lymphocyte tubes from Nunc (Roskilde, Denmark) at 106 cells/ml using RPMI 1640 medium containing 0.01 m HEPES buffer, 0.2 m glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin (Gibco, Paisley, UK) and 10% fetal calf serum (FCS; HyClone Labs, Logan, UT). Stimulation with pokeweed mitogen (PWM; Sigma, St Louis, MO) was carried out at 1 μg/ml. In experiments measuring immunoglobulin production, hydrocortisone (Sigma) was added at 10−5m.
Immunoglobulin production in vitro
Immunoglobulin levels in cell-free culture supernatants were measured after 2, 4, 6, 8 and 14 days of culture using the ELISA method described by Sigfússon et al. [11].
B and T cell subpopulations
B and T cell subpopulations were analysed by flow cytometry using the Becton Dickinson FACScan and directly labelled MoAbs to: CD3, CD4, CD8, CD5, CD10, CD11b, CD19 and CD38 (Becton Dickinson, San Diego, CA). In paraffin-embedded tissue sections B cells were identified using antibody against CD20 (clone L26) from Dako A/S (Glostrup Denmark).
Cell survival in culture
Cells were cultured as described above. After 2, 8 and 14 days in culture aliquots were removed for haemocytometer counting using trypan blue exclusion to indicate viability. The percentage of surviving cells on day 14 was calculated as: (number of cells alive on day 14/number of cells alive on day 2) × 100.
Expression of Bcl-2 protein
Mouse monoclonal anti-Bcl-2 (clone 124) was obtained from Dako and used unlabelled for immunohistochemistry on 4-μm paraffin-embedded tissue sections. After deparaffinization and rehydration the sections were heated in 0.01 m citrate buffer for twice 5 min in a microwave oven for antigen retrieval. Staining was visualized using Vectastain ABC Kit (Vector Labs, Burlingame, CA). The same antibody was used directly FITC-labelled for flow cytometry of fresh or cultured peripheral blood mononuclear cells (PBMC) following the manufacturer's protocol. For double staining of a surface marker and Bcl-2 the cells were incubated with RPE-labelled antibody against the surface antigen and then fixed in 1 ml of 0.25% formaldehyde added slowly while vortexing and incubated in the dark for 15 min at room temperature. After washing once with PBS the cells were permeabilized in 1 ml of cold (4°C) 70% methanol for 60 min at 4°C. In the cultures the cells were initially depleted of monocytes by adherence to 25-cm2 culture flasks (Nunc) for 90 min. This prevented the loss of apoptotic cells as verified by preliminary experiments.
Detection of apoptosis
Apoptosis was detected using the TUNEL assay for flow cytometry (Boehringer-Mannheim, Mannheim, Germany) according to the manufacturer's instructions.
RESULTS
Subpopulation analysis of B and T cells
In order to test for possible differences in the proportions of B cell subsets, PB lymphocytes were analysed by flow cytometry for expression of CD5, CD10, CD11b and CD38 along with CD19 as a B cell marker. Unstimulated cells from seven hyper-responders and seven normal responders from the family were tested and compared with cells from 26 healthy donors. No differences were observed between cells from family members and unrelated controls, and the proportions of peripheral blood B cells expressing CD5, CD11b and CD38 were 27.6%, 4.2% and 57.1%, respectively, for all groups combined, whereas CD10 was undetectable. Subpopulations of T cells expressing CD4 or CD8 were analysed during 6–14 days of culture with or without mitogen on two separate occasions. During the 6-day culture with mitogen no differences were detectable between family members and controls. The means for both groups showed an increase in the proportion of CD3+ T cells from 64% to 83% and a decline in B cells from 15% to 6%. The ratio of CD4 to CD8 cells increased slightly with culture in both groups. During the 14-day culture there were no differences in relative cell numbers in the unstimulated cultures. In the stimulated cultures, however, no viable B cells could be detected at the end of the culture period in any of eight control cultures, whereas five out of six cultures from hyper-responders contained a small proportion of B cells (0.8–2.6%). There was therefore a suggestion of enhanced survival of B cells but no evidence of differences in the pattern of T cell subsets that might suggest deficiencies in T cell-operated control of B cells. In fact, the lowest individual CD4/CD8 ratios were found among the hyper-responders.
Time course and dose dependence of immunoglobulin production in response to mitogen stimulation
Seven of the 10 family members originally defined as hyper-responders were retested once or twice. Although there was some variability between test occasions, five subjects consistently showed high levels, including a mother and two of her children. In our first experiments the measurements were performed after 7 days of culture. It was thus possible either that the initiation of response was more vigorous in lymphocytes from the hyper-responders, or that their response was sustained for longer. Immunoglobulins were measured in culture supernatants after 2, 4, 6, 8 and 14 days of culture on two separate occasions. The main results are shown in Fig. 1. The initial rate of response was the same for five hyper-responder family members and controls. Thus IgG production was first detectable on day 4 and the levels were very similar in hyper-responder cultures and control cultures; 0.84 μg/ml (range 0.34–1.66 μg/ml) and 0.41 μg/ml (range 0.18–1.32 μg/ml), respectively. In the longer-term cultures, differences were observed, with IgG levels increased over controls on day 8 and continuing to rise up to 14 days of culture in the hyper-responders, whilst in cultures from normal responders or control subjects the levels peaked on day 8 (Fig. 1). Cultures were also tested with a lower dose of PWM (0.1 μg/ml). This produced erratic stimulation and there was no evidence of higher sensitivity among the hyper-responders (results not shown).
Fig. 1.
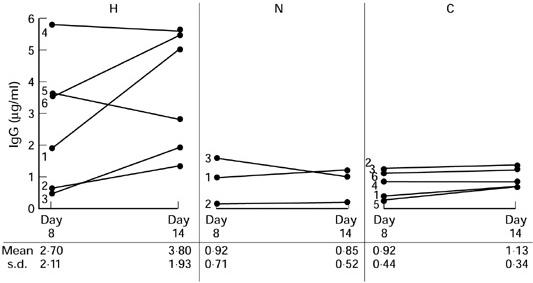
In vitro production of IgG in cultures from six hyper-responders (H) and three normal responders (N) from the family and six unrelated control subjects (C), stimulated with 1 μg/ml of pokeweed mitogen (PWM).
Lymphocyte survival during 14 days of culture with and without mitogen
Having established that the abnormally high production of immunoglobulins was associated with a longer-lasting response rather than differences in the initiation, it was of interest to monitor the survival of lymphocytes in culture. After an initial proliferative response in cultures exposed to PWM these cultures showed a considerably higher death rate than unstimulated cultures. The proportion of surviving cells after 14 days of culture with PWM compared with day 2 is shown in Table 1. Cultures from hyper-responders showed significantly higher proportionate cell survival than cultures from normal responders from the family or unrelated control subjects. One hyper-responder (no. 2) did not show increased cell survival, and IgG production in this sample was lower than on two previous occasions. This was the oldest family member tested, 89 years at the time of third testing. As already noted above, surviving B cells were only present in 14-day stimulated cultures from hyper-responders.
Table 1.
Lymphocyte survival in cultures stimulated with pokeweed mitogen (PWM)
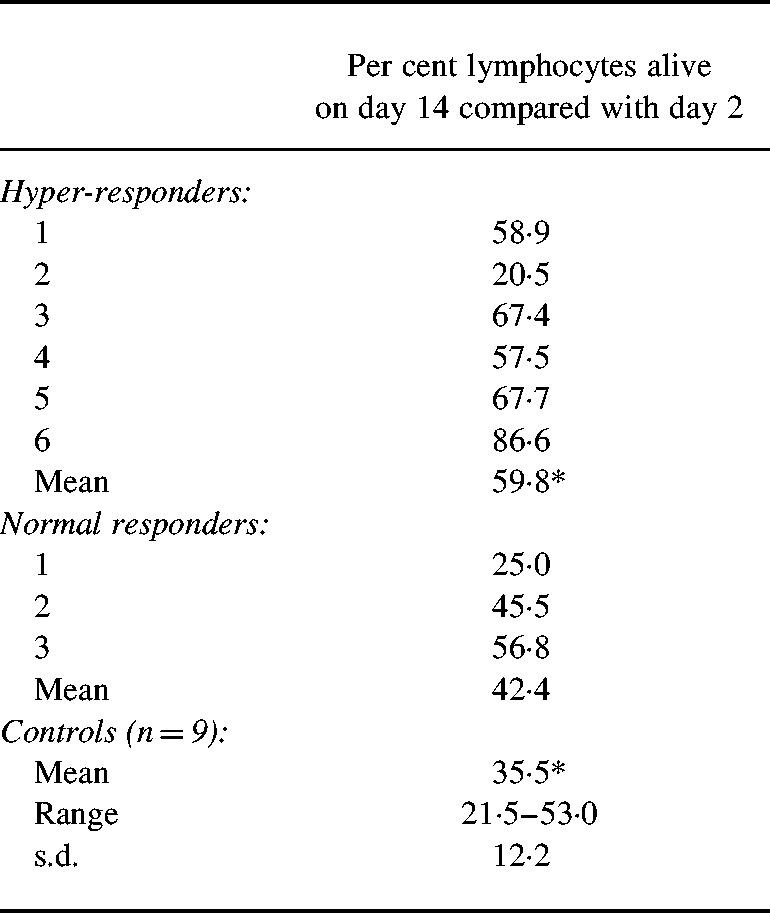
The percent cell survival was calculated as described in Subjects and Methods. The individual subjects are numbered as in Fig. 1. Statistics: two-tailed Student's t-test.
*P = 0.016.
Expression of Bcl-2 protein in tissue samples and cultured lymphocytes
In the last part of this study we looked for evidence that the enhanced B cell survival was associated with increased expression of Bcl-2. Archival tissue samples were available from the lymphoid neoplasias that have been diagnosed in this family. Bcl-2 was strongly expressed in the immunoblastic lymphoma and bone marrow biopsies from the case of Waldenström's macroglobulinaemia, and the case of multiple myeloma showed more scattered expression. The pattern of expression was identical to that seen in sections from unrelated control patients with the same diagnoses. From the family member who developed multiple myeloma a lymph node had been removed because of tuberculosis 23 years previously. As shown in Fig. 2b), this lymph node showed marked expression of Bcl-2 in a B cell follicle. For comparison, normal follicles revealed very little Bcl-2 expression in B cell follicles (Fig. 2a), in contrast to the strong expression characteristic of T cell areas. The same was true of a tuberculous lymph node from an unrelated patient (Fig. 2c), demonstrating that Bcl-2 expression in lymphocytes is not influenced by the mycobacteria. The identity of lymphocytes as B cells was confirmed by staining adjacent tissues sections with antibody against CD20.
Fig. 2.
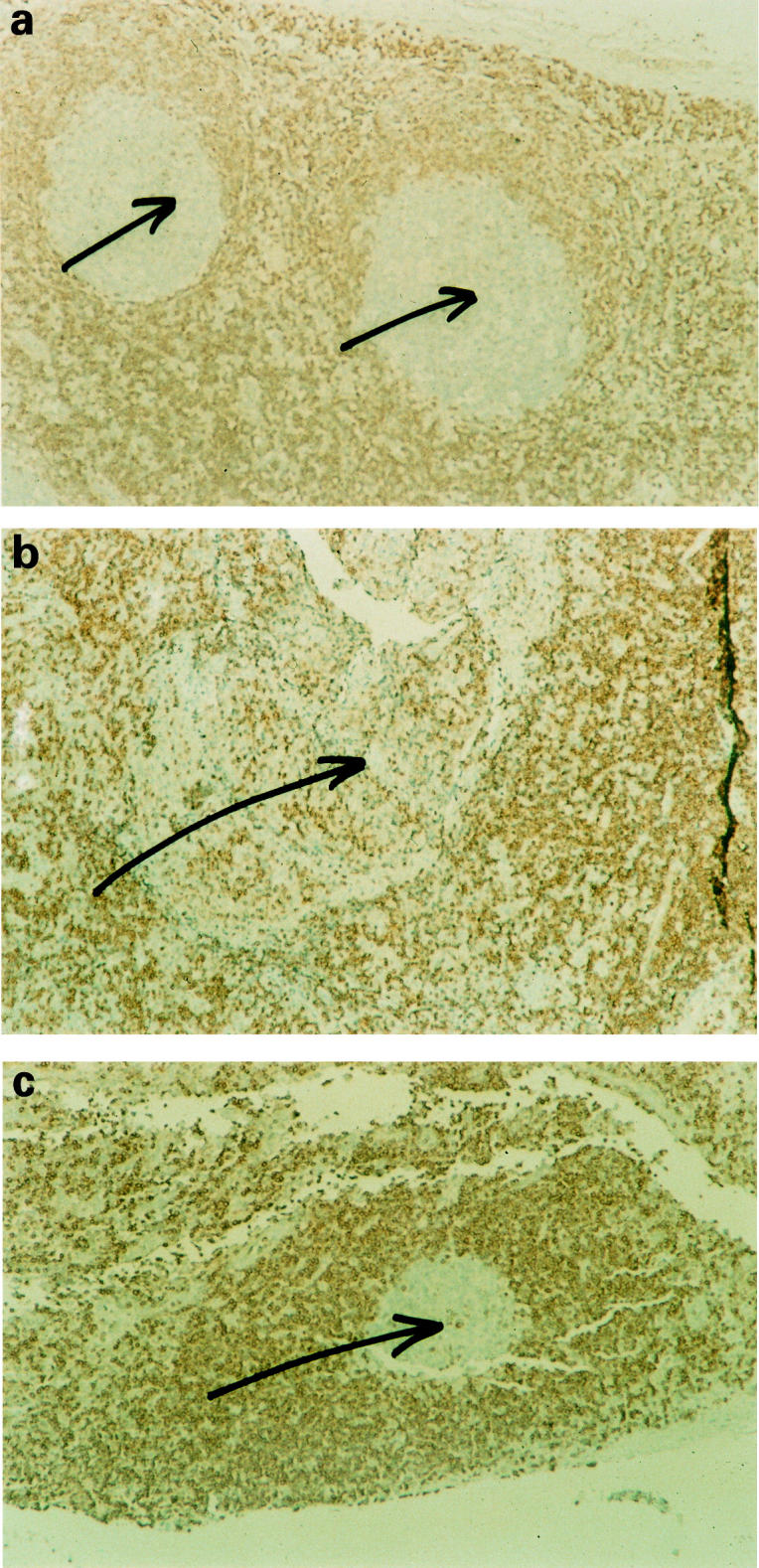
Immunohistochemical staining for Bcl-2 protein in (a) normal lymph node, (b) tuberculous lymph node from family member, and (c) tuberculous lymph node from unrelated patient. Note the marked expression of Bcl-2 in the B cell follicle (b) compared with (a) and (c) (arrows). (Original mag. × 125.)
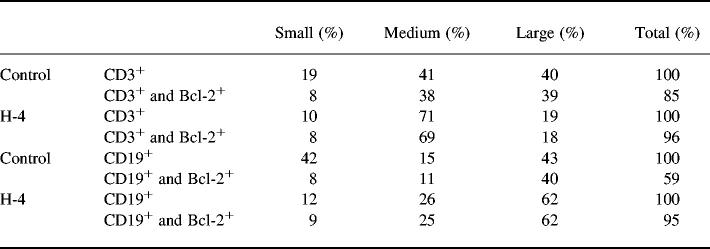
The expression of Bcl-2 was analysed by flow cytometry on two separate occasions in unstimulated and PWM-stimulated cultures of PB lymphocytes. In freshly isolated lymphocytes Bcl-2 was expressed in 88% (range 77–93%) of B cells and 96% (range 83–99%) of T cells in a group of control subjects. The expression of Bcl-2 changed with time in several control cultures tested, showing an initial rise to almost 100%, followed by a marked decline after > 48 h of culture particularly in the presence of PWM. At the same time the proportion of apoptotic cells among medium-sized lymphocytes increased to around 50% (at 48 h) and around 90% (at 192 h), as judged by TUNEL staining. Table 2 shows the size distribution of T and B lymphocytes after 4 days of cluture with PWM and the Bcl-2 expression in each size group as analysed for one hyper-responder (H-4) and one control subject. The picture for T cells was similar, although there was a higher proportion of large blasts in the control culture. For the B cells the proportion of small, dying cells was much lower in the sample from H-4 and all size groups show retention of Bcl-2.
Table 2.
Size distribution and Bcl-2 expression of lymphocytes after 4 days of culture with pokeweed mitogen (PWM)
Analysed from flow cytometry by gating the cells into small, medium sized and large cells that formed distinct subgroups on the scattergrams. The size distribution is shown for T and B cells separately and the fraction of Bcl-2+ cells within each subgroup.
In the second series two other hyper-responders and one normal responding family member were tested along with five control subjects. Cultures from the two hyper-responders contained 9.9% (H-5) and 16.6% (H-3) Bcl-2+ B cells (CD19+) after 6 days of culture with PWM (total ungated cell population). This population was 5.4% (range 2.9–8.4%, s.d. 2.2) in five control subjects who showed an equivalent level of proliferative response. The one normal-responding family member was within the normal range with 7.4% of the stimulated cell population co-expressing Bcl-2 and CD19. Hyper-responders did not differ from controls in the Bcl-2 expression of B cells before stimulation; the proportion of Bcl-2+ B cells was 6.9% (C), 7.4% (H) and 7.8% (N). Figure 3 shows one of these experiments in further detail, analysed with respect to Bcl-2+ cells. It is clear that the hyper-responder was not different from the controls at the beginning of the culture but the difference became apparent with increasing time in culture when the control cells started showing a decline in Bcl-2 expression and cell death. The prolonged retention of Bcl-2 in the hyper-responder cells compared with controls was specific for B cells (Fig. 3b).
Fig. 3.
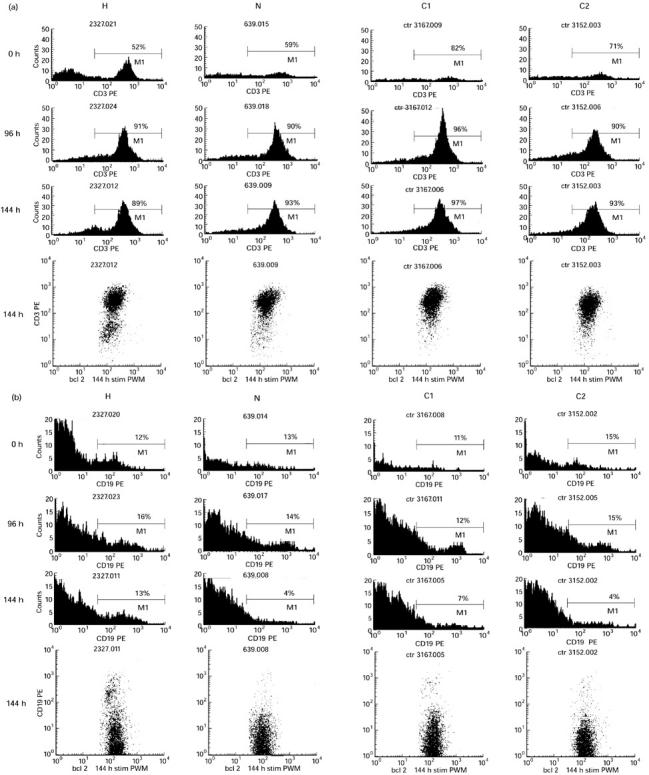
Cells were gated for Bcl-2+ cells. Histograms show the proportion of Bcl-2+ cells expressing (a) the T cell marker CD3, and (b) the B cell marker CD19 (see next page). C1 and C2, two control subjects; H, hyper-responder; N, normal responding family member. Note the marked difference in Bcl-2-expressing B cells at 144 h.
DISCUSSION
The family described here has been under study for 20 years. This long study period is, of course, reflected in the work. On the one hand knowledge and technology have advanced, on the other hand family members have died or been unavailable for repeated sampling for other reasons, and the younger family members have provided fewer samples than the older generations. The first observations were on clinical manifestations of clonal B cell disorders and polyclonally raised serum levels of IgM in otherwise healthy family members [8]. Later it was demonstrated that cultured B cells from some family members produced increased amounts of immunoglobulins of all classes upon mitogenic stimulation and they were identified as hyper-responders [10]. In the current study we have obtained evidence that this abnormally high immunoglobulin production is associated with prolonged survival of B cells and enhanced expression of Bcl-2 after stimulation. No abnormalities were detected in B cell phenotype using markers that have been associated with Waldenström's macroglobulinaemia [12] or that are characteristic of differentiation stages of B cells [13]. The time course of immunoglobulin production showed that there was no indication of accelerated B cell triggering, and intact immunoglobulin class switching implies normal control by T cells. This was further supported by the changes seen in the proportions of CD4+ and CD8+ T cells during culture. Previous studies had shown that the proliferative response of lymphocytes from the hyper-responders to mitogenic stimulation was not increased and natural killer (NK) cell function was normal [10]. Thus the basic functional abnormality in this family is an extended life span of B cells following stimulation with prolonged expression of Bcl-2.
Bcl-2 was first described as the protein over-expressed in B cell-derived malignancies as a consequence of the t14:18 translocation which brings the bcl-2 gene into proximity with the immunoglobulin heavy chain locus [7,14,15]. It was soon realized that Bcl-2 is also expressed in normal cells and is a hallmark of long-lived T cells and B cells [7,15–18]. The requirement for Bcl-2 for lymphocyte survival is demonstrated by the fulminant apoptotic loss of mature lymphocytes in Bcl-2-deficient mice [19]. It has since become clear that Bcl-2 is but one member of a large family of molecules that are engaged in the control of cellular life and death [4,5]. Some of the more recently described Bcl-2-related molecules have also been implicated in B cell longevity, including Bcl-x [20] and A1 [21]. We have not had the opportunity to test for the involvement of these Bcl-2-related proteins in the family described here.
Bcl-2 expression of freshly isolated unstimulated peripheral blood B cells was not noticeably higher in samples from hyper-responders compared with unrelated controls or normal responder family members. The prolonged increase in Bcl-2 expression was thus a secondary phenomenon. The enhanced Bcl-2 expression in B cells in the tuberculous lymph node of the patient who subsequently developed multiple myeloma was particularly remarkable, as this is not a known feature of reactive B cell follicles. It is not likely that this indicated the presence of a malignant clone of B cells since 23 years passed until the multiple myeloma was diagnosed. This family member had also been investigated 9 years before the multiple myeloma was diagnosed and showed then a slight polyclonal rise in serum IgM but no evidence of a monoclonal paraprotein [8]. Bone marrow biopsies of the multiple myeloma showed only moderate expression of Bcl-2 in the malignant cells. Since the original description of the t14:18 translocation in B cell lymphomas it has now been realized that this translocation can also be detected in the peripheral blood of normal blood donors [22]. It is thus conceivable that the retained Bcl-2 expression in B cells of hyper-responders following stimulation in vitro was caused by selection in culture of cells harbouring this translocation. This would imply a higher frequency of t14:18 in the hyper-responders. There is, however, no evidence of chromosomal instability in these family members judging from karyotypings on freshly isolated lymphocytes or Epstein–Barr-infected B cell lines (unpublished results). The frequency of t14:18 has been noted to increase with age [23]. Increased expression of Bcl-2 was confirmed in family members aged from 21 to 68 years.
No clear picture has emerged as yet of the physiological control of Bcl-2 or other related proteins. Since Bcl-2-type proteins are present in a wide variety of cell types, control mechanisms are likely to depend on different growth factors and differentiation factors according to cell type. Thus it has been shown that Bcl-2 is up-regulated by IL-5 in eosinophils [24] and IL-15 in NK cells [25]. In T cells IL-2 and IL-10 have been associated with up-regulation [26,27] and IL-6 prevented down-regulation [28]. In normal tonsillar B cells IL-10 directed differentiation into plasma cells and down-regulation of Bcl-2 [29,30], whereas CD40-L with IL-2 induced Bcl-2 and maintained proliferation and survival [29].
Inappropriate escape from apoptosis in the immune system is associated with two types of pathology, lymphoproliferative diseases (benign or malignant) and autoimmunity [3,31–34]. Mutations leading to lack of functional Fas result in an increased life span of T cells and are associated with autoimmunity and lymphoproliferative disease in mice and men [3]. The clinical picture in man, the so-called Canale–Smith syndrome, consists mainly of widespread lymphoproliferation in childhood, that appears to be usually oligiclonal and benign, with occasional autoimmunity [35,36]. Mice with an immunoglobulin Bcl-2 transgene have extended B cell survival and show polyclonal follicular lymphoproliferation [37]. In the context of the present study it is of interest to note that CD5+ cells were not over-represented among the expanded B cell population, and in vitro proliferative responses to mitogens were normal on day 3 of culture although they appeared to be increased on day 5, which was attributed to enhanced cell survival [38]. Recently it was demonstrated that Bcl-2 over-expression can block self-tolerance in mature peripheral B cells [39]. The Bcl-2 transgenic mice develop B cell lymphomas when they get old [40]. The lymphomagenesis was considerably accelerated in mice that were doubly transgenic for Bcl-2 and the myc oncogene [6]. Over-expression of Bcl-2 alone is thus not a very potent oncogenic stimulus. Familial macroglobulinaemia has in some families been associated with autoimmunity [9]. In the family described here this has not been the case and the most common clinical presentation has been benign monoclonal gammopathy (monoclonal gammopathy of undetermined significance (MGUS)) fairly late in life with paraprotein-related symptoms and late onset B cell malignancies.
In conclusion, the family described here shows an inherited tendency to increased and prolonged secretion of immunoglobulins as a result of increased B cell survival that is associated with increased expression of Bcl-2. The clinical picture shows certain similarities to that observed in mice with transgenic over-expression of Bcl-2, including an increased risk of developing B cell-derived malignancies. The over-expression of Bcl-2 is probably a secondary phenomenon and further studies are in progress in order to elucidate the mechanisms of the aberrant control. The understanding that can be gained from the study of rare human disorders is likely to provide an insight into mechanisms involved in normal physiological control.
Acknowledgments
The financial support of the Research Fund of the University of Iceland is gratefully acknowledged. We also thank Helga Kristjánsdóttir and Kristrún Ólafsdóttir for their skilful technical assistance. The helpful comments of Dr Thorunn Rafnar on the manuscript were greatly appreciated.
REFERENCES
- 1.Green DR, Scott DW. Activation-induced apoptosis in lymphocytes. Curr Opin Immunol. 1994;6:476–87. doi: 10.1016/0952-7915(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 2.Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin KM. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994;6:279–89. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 3.Rathmell JC, Goodnow CC. The Fas track. Curr Biol. 1995;5:1218–21. doi: 10.1016/s0960-9822(95)00241-7. [DOI] [PubMed] [Google Scholar]
- 4.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–6. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature Med. 1997;3:614–20. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 6.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–3. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 7.Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80:879–86. [PubMed] [Google Scholar]
- 8.Björnsson ÓG, Árnason A, Guðmundson S, Jensson Ó, Ólafsson S, Valdimarsson H. Macroglobulinaemia in an Icelandic family. Acta Med Scand. 1978;203:283–8. doi: 10.1111/j.0954-6820.1978.tb14874.x. [DOI] [PubMed] [Google Scholar]
- 9.Renier G, Ifrah N, Chevalier A, Seint-Andre JP, Boasson M, Hurez D. Four brothers with Waldenström's macroglobulinaemia. Cancer. 1989;64:1554–9. doi: 10.1002/1097-0142(19891001)64:7<1554::aid-cncr2820640734>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Ögmundsdóttir HM, Jóhannesson GM, Sveinsdóttir S, Einarsdóttir S, Hegeman A, Jensson Ó. Familial macroglobulinaemia: hyperactive B-cells but normal natural killer function. Scand J Immunol. 1994;40:195–200. doi: 10.1111/j.1365-3083.1994.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 11.Sigfússon Á, Babbage JW, Souhami RL. Defective in vitro antibody production in response to pokeweed mitogen and influenza antigen in patients with Hodgkin's disease. Clin Exp Immunol. 1985;60:396–402. [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen GS, Andrews EJ, Mant MJ, Vergidis R, Ledbetter JA, Pilarski L. Transitions in CD45 isoform expression indicate continuous differentiation of a monoclonal CD5+ CD11b+ B lineage in Waldenström's macroglobulinemia. Am J Hematol. 1991;37:20–30. doi: 10.1002/ajh.2830370106. [DOI] [PubMed] [Google Scholar]
- 13.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–26. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 14.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–9. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 15.Pezzella F, Tse AGD, Cordell JL, et al. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990;137:225–32. [PMC free article] [PubMed] [Google Scholar]
- 16.Gratiot-Deans J, Ding L, Turka LA, Nuñez G. bcl-2 proto-oncogene expression during human T cell development. Evidence for biphasic regulation. J Immunol. 1993;151:83–91. [PubMed] [Google Scholar]
- 17.Petterson M, Jernberg-Wiklund H, Larsson L-G, Sundström C, Givol I, Tsujimoto Y, Nilsson K. Expression of the bcl-2 gene in human multiple myeloma cells lines and normal plasma cells. Blood. 1992;79:495–502. [PubMed] [Google Scholar]
- 18.Kondo E, Yoshino T, Nomura S, Nakamura S, Takahashi K, Teramoto N, Hayashi K, Akagi T. bcl-2 regulation in normal resting lymphocytes and lymphoblasts. Jpn J Cancer Res. 1994;85:260–5. doi: 10.1111/j.1349-7006.1994.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–40. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 20.Tuscano JM, Druey KM, Riva A, Pena J, Thompson CB, Kehrl JH. Bcl-x rather than Bcl-2 mediates CD40-dependent centrocyte survival in the germinal center. Blood. 1996;88:1359–64. [PubMed] [Google Scholar]
- 21.Tomayko MM, Cancro MP. Long-lived B cells are distinguished by elevated expression of A1. J Immunol. 1998;160:107–11. [PubMed] [Google Scholar]
- 22.Dolken G, Illerhaus G, Hirt C, Mertelsmann R. BCL-2/JH rearrangements in circulating B cells of healthy blood donors and patients with nonmalignant diseases. Clin Oncol. 1996;14:1333–44. doi: 10.1200/JCO.1996.14.4.1333. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Hernandez AM, Shibata D, Cortopassi GA. BCL2 translocation frequency rises with age in humans. Proc Nat Acad Sci USA. 1994;91:8910–4. doi: 10.1073/pnas.91.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochiai K, Kagami M, Matsumura R, Tomioka H. IL-5 but not interferon-gamma (IFN-gamma) inhibits eosinophil apoptosis by up-regulation of bcl-2 expression. Clin Exp Immunol. 1997;107:198–204. doi: 10.1046/j.1365-2249.1997.d01-884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carson WE, Fehniger TA, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–43. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mor F, Cohen IR. IL-2 rescues antigen-specific T cells from radiation or dexamethasone-induced apoptosis. Correlation with induction of Bcl-2. J Immunol. 1996;156:515–22. [PubMed] [Google Scholar]
- 27.Cohen SBA, Crawley JB, Kahan MC, Feldmann M, Foxwell BMJ. Interleukin-10 rescues T cells from apoptotic death: association with an upregulation of Bcl-2. Immunology. 1997;92:1–5. doi: 10.1046/j.1365-2567.1997.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues mouse T cells from apoptosis. J Immunol. 1997;158:5791–6. [PubMed] [Google Scholar]
- 29.Choe J, Kim H-S, Zhang X, Armitage R, Choi YS. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. J Immunol. 1996;157:1006–16. [PubMed] [Google Scholar]
- 30.Li L, Krajewski S, Reed JC, Choi YS. The apoptosis and proliferation of SAC-activated B cells by IL-10 are associated with changes in Bcl-2, Bcl-xL, and Mcl-1 expression. Cell Immunol. 1997;178:33–41. doi: 10.1006/cimm.1997.1129. [DOI] [PubMed] [Google Scholar]
- 31.MacLennan ICM. Avoiding autoreactivity. Nature. 1995;375:281. doi: 10.1038/375281a0. [DOI] [PubMed] [Google Scholar]
- 32.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–61. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 33.Caligaris-Cappio F, Ferrarini M. B cells and their fate in health and disease. Immunol Today. 1996;17:206–8. doi: 10.1016/0167-5699(96)30008-x. [DOI] [PubMed] [Google Scholar]
- 34.Salmon M, Pilling D, Borthwick NJ, Akbar AN. Inhibition of T-cell apoptosis—a mechanism for persistence in chronic inflammation. The Immunologist. 1997;5:87–92. [Google Scholar]
- 35.Rieux-Leucat F, Le Deist F, Hivroz C, Roberts IAG, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–9. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 36.Drappa J, Vaishnaw AK, Sullivan KE, Chu J-L, Elkon KB. Fas gene mutation in the Canale–Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. New Engl J Med. 1996;335:1643–49. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- 37.McDonnell TJ, Deane N, Platt FM, Nuñez G, Jaeger U, McKearn JP, Korsmeyer SJ. Bcl-2-transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 38.Yeh T-M, Korsmeyer SJ, Teale JM. Skewed B cell VH family repertoire in Bcl-2-Ig transgenic mice. Int Immunol. 1991;3:1329–33. doi: 10.1093/intimm/3.12.1329. [DOI] [PubMed] [Google Scholar]
- 39.Lang J, Arnold B, Hammerling G, Harris AW, Korsmeyer S, Russell D, Strasser A, Nemazee D. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J Exp Med. 1997;186:1513–22. doi: 10.1084/jem.186.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18) Nature. 1991;349:254–6. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]