Abstract
Expression of chemokine receptors and β-chemokine production by peripheral blood mononuclear cells (PBMC) were determined in HIV-1-infected individuals before and after highly active anti-retroviral therapy (HAART) and their relationship to viral load, T cell phenotype and the expression of immunological activation markers was examined. We found that the expression of CCR5 is up-regulated in HIV-1-infected individuals while CXCR4 appears down-regulated on both CD4 and CD8 T cells compared with normal controls. These alterations are associated with the high levels of viral load. In addition, a relationship was observed between the degree of immune activation and chemokine receptor expression on T cells. However, after 3 months of combined anti-retroviral regimen, expression of CXCR4 significantly increased while CCR5 decreased when compared with pretherapy determinations. This was seen in strict association with a dramatic decrease of viral load and an increase of both CD45RA+/CD62L+ (naive) and CD45RA−/CD62L+ or CD45RA+/CD62L− (memory) T cells accompanied by a significant decrease of the expression of immune activation markers such as HLA-DR and CD38. At enrolment, both spontaneous and lectin-induced RANTES, macrophage inflammatory protein-1α (MIP-1α) and MIP-1β production by PBMC were higher in HIV-1-infected individuals compared with normal controls, although differences for MIP-1β were not statistically significant. However, RANTES and MIP-1α production decreased during HAART at levels closer to that determined with normal controls, while MIP-1β production was less consistently modified. These data indicate that the expression of chemokine receptors CCR5 and CXCR4 and the production of β-chemokines are altered in HIV-infected individuals, and suggest that their early modifications during HAART reflect both the peripheral redistribution of naive/memory T cell compartments and the decrease in levels of T cell activation. Such modifications in the expression of host determinants of viral tropism and the production of anti-viral molecules may play a role in the emergence of virus variants when a failure of HAART occurs.
Keywords: chemokine receptors, β-chemokines, HIV, HAART
INTRODUCTION
Chemokine receptors are used by HIV-1 for entry into CD4 T cells [1–6]. During the early stages of disease, HIV-1 isolates are preferentially monocytotropic with a non-syncytium-inducing phenotype and use CCR5 as a co-receptor [7–9]. CCR5-expressing CD4 cells also express CD45RO and CD95, a characteristic phenotype of memory T cells [10–12]. In contrast, progression of disease is associated with the emergence of syncytium-inducing viruses that are able to bind the CXCR4 chemokine receptor [7]. Persistence of CCR5 usage is seen in association with delayed progression of disease, such as that observed in long-term non-progressors (LTnPs) [9]. Recently, Ostrowski et al. demonstrated that CXCR4 is down-regulated while CCR5 expression is up-regulated in T cells of HIV-1-infected individuals compared with uninfected controls [13].
Highly active anti-retroviral therapy (HAART) results in a rapid decline of viral load to undetectable levels and an increase of CD4 T cell count [14], and is often associated with an improvement of clinical and immunological status [15]. In fact, several immune defects that characterize HIV-1 infection are mitigated by anti-retroviral therapy and in some patients an initial immune reconstitution has been observed [16–18]. However, no data are available at present about the effect of HAART on the expression of chemokine receptors and the production of β-chemokines which may act in controlling viral entry into target cells. In this study we investigated the expression of CXCR4 and CCR5 as well as the spontaneous and lectin-induced production of RANTES, macrophage inflammatory protein-1α (MIP-1α) and MIP-1β in HIV-1-infected individuals before and after HAART. We also examined the relationship with immunological and virological parameters, including the distribution of naive and memory cells and the levels of expression of immune activation markers.
PATIENTS AND METHODS
Patients
Seventeen HIV-1+ patients from our department were enrolled in an open prospective study with HAART. Main inclusion criteria were: CD4+ T cell count between 100 and 500/μl, HIV-RNA plasma viraemia > 10 000 copies/ml. All patients were naive for protease inhibitor therapy. Of the 17 patients, 11 were males and six females. The median age at entry was 36.2 years (range 26–56 years). According to risk factors, the patients were stratified as follows: seven heterosexual, five homo-bisexual, and five intravenous drug users. Mean value of CD4+ T cell count on entry was 323/μl (range 181–475/μl). Mean value of plasma HIV-1-RNA was 4.62 ± 0.5 log10(range 3.9–5.85). Ten subjects were nucleoside reverse transcriptase inhibitors (NRTI)-experienced (mean previous therapy with NRTI was 1.4 years). No concomitant active opportunistic infections were present at enrolment. Karnofsky's score, clinical signs and symptoms of HIV-related and AIDS-defining events were monitored monthly. Blood samples were taken on entry and every 4 weeks for blood chemistry and haematological evaluations. Immunologic and virologic determinations were performed after 3 months. All subjects gave informed written consent according to the University Ethical Committee procedures.
Monoclonal antibodies
Four-colour cytofluorometric analyses were performed using the following MoAbs: anti-CD4–allophycocyanin (APC), anti-CD8–peridinin chlorophyll protein (PerCP), anti-CD45RA–FITC, anti-HLA-DR–PE, and anti-CD38–PE, obtained from Becton Dickinson (San Jose, CA); anti-CD62L–PE, anti-CCR5–PE clone 2D7 and anti-CXCR4–PE clone 12G5, obtained from PharMingen (San Diego, CA); anti-Fas–FITC obtained from MBL (Medical & Biological Laboratories Co., Ltd., Nagoya, Japan).
Flow cytometry
Flow cytometric analysis was performed with freshly collected blood samples from HIV-1-infected individuals or normal blood donors at enrolment (time 0) and after 3 months (time 3) of HAART regimen. In some experiments, frozen samples of peripheral blood mononuclear cells (PBMC) were used. In these cases, frozen PBMC samples were thawed rapidly in a 37°C water bath, washed twice in RPMI–20% fetal calf serum (FCS) and resuspended in PBS–2% FCS. The cells were stained at 4°C for 30 min with a cocktail of four MoAbs. After staining cells were washed once in PBS and directly subjected to cytofluorometric analysis performed by a FACS Calibur (Becton Dickinson) equipped with Cell Quest (Macintosh) software [19]. To determine co-receptor expression on CD4+ and CD8+ cells, total lymphocytes were first identified and gated by forward and side scatter. The cells were then additionally gated for CD4+ or CD8+ expression. The resulting bivariate plots of CCR5 and CXCR4 staining were then analysed according to the isotype-matched controls. The following combinations were analysed: (i) anti-Fas–FITC, anti-CCR5–PE, anti-CD8–PerCP, anti-CD4–APC; (ii) anti-CXCR4–PE, anti-CD8–PerCP, anti-CD4–APC. The naive/memory phenotype was studied apart from co-receptor expression by incubating PBMC with a mixture of anti-CD45RA–FITC, anti-CD62L–PE, anti-CD8–PerCP, anti-CD4–APC.
Determination of viral load
Plasma HIV RNA levels were determined using the Roche Amplicor assay following the manufacturer's instructions. In patients with viral load < 200, an ultrasensitive assay procedure was performed. This procedure, designed for use with the Amplicor HIV-1 Monitor Test, increases the analytical sensitivity of the test, thereby permitting lower levels of HIV-1-RNA to be quantified [20,21]. The increased sensitivity is obtained by (i) increasing the specimen volume (from 200 μl to 500 μl); (ii) performing high speed centrifugation of virus particles (24 000 g for 60 min at 2–8°C); (iii) reducing the volume of specimen diluent used to resuspend the pelleted virus (100 μl rather than 400 μl). Such modification of the specimen preparation protocol permitted detection of as few as 20 viral copies/ml.
β-chemokine production
PBMC short-term cultures and conditioned medium (CM) preparations were performed with freshly collected blood samples from HIV-1-infected individuals or normal blood donors at enrolment (time 0) and after 3 months (time 3).
PBMC were isolated from whole blood by Ficoll–Hypaque gradient centrifugation, counted and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) in 5% CO2 atmosphere at 37°C. CM was prepared from either unstimulated or lectin-activated PBMC from HIV-1-infected individuals and normal donors as previously described [22]. Briefly, 1 × 106 cells were cultured in the absence of stimuli to verify the spontaneous production of chemokines. Alternatively, PBMC were activated by incubation with phytohaemagglutinin (PHA) at 1 μg/ml concentration. CM from unstimulated cells was then collected at 72 h and CM from lectin-activated PBMC after 48 h of culture, respectively, centrifuged at 1500 g at 4°C for 30 min, and tested for chemokine content. To avoid loss of chemokines, all samples were handled in plasticware precoated with PBS 0.1% bovine serum albumin (BSA). Measurement of RANTES, MIP-1α and MIP-1β production was performed with PBMC supernatants from both HIV-1-infected individuals and normal controls by ELISA according to manufacturer's instructions (R&D Systems, Minneapolis, MN).
RESULTS
Expression of CCR5 and CXCR4 by CD4 and CD8 T cells from HIV-1-infected and uninfected individuals
PBMC from 17 HIV-1-infected patients were analysed for the expression of CCR5 and CXCR4 before and after 3 months of therapy. In addition, CCR5 and CXCR4 expression was determined in PBMC samples from 10 uninfected, healthy blood donors which represented normal controls. In some instances frozen PBMC samples from HIV-1-infected patients were used, after determining that CCR5 and CXCR4 expression levels on freshly isolated and cryopreserved PBMC from the same donor were not significantly different. In particular, the frequencies of CCR5-expressing CD4+ T cells from five HIV-1-infected patients (at enrolment and during HAART) were 26%, 22%, 16%, 14% and 10%, respectively, on fresh cells and 25%, 21%, 16%, 14% and 10% on the corresponding frozen PBMC sample (P = 0.2). Similarly, the percentages of CXCR4-expressing CD4 T cells were 51%, 57%, 59%, 65% and 67% in fresh samples and 52%, 59%, 60%, 63% and 66% in frozen samples, respectively (P = 0.8). Comparable results were observed for CCR5 and CXCR4 expression on CD8+ T cells.
The expression of CCR5 in HIV-infected patients before therapy was significantly increased in both CD4+ and CD8+ T cells in comparison with normal controls (for CD4+ cells: mean 17.3 ± 5.5% versus 12.2 ± 1.8%; P = 0.014; for CD8+ cells: mean 51.5 ± 15% versus 26.8 ± 7.3%; P = 0.0001). On the other hand, the expression of CXCR4 in the same cell types was clearly down-regulated compared with uninfected controls (for CD4+ cells: mean 61.8 ± 18.3% versus 83.3 ± 2.5%; P = 0.0012; for CD8+ cells: mean 42 ± 24.9% versus 77.3 ± 5.6%; P = 0.0002). The altered expression of CCR5 and CXCR4 was particularly evident in CD8+ T cells when compared with that of CD4+ T cells.
High levels of T cell activation, evaluated by HLA-DR, CD38 and Fas antigen determination, were found in HIV-1-infected individuals when compared with uninfected controls. As expected, higher levels of T cell activation were observed in CD8+ T cells. A statistically significant correlation was observed between percentage of CD4+ cells and HLA-DR expression (r = 0.645; P = 0.0069). However, the increased expression of CCR5 in CD4+ T cells was only weakly related to HLA-DR expression (r = 0.463; P = 0.071).
After 3 months of therapy the expression of CCR5 and CXCR4 was significantly modified in both CD4+ and CD8+ T cells in the majority of the patients investigated (Fig. 1a–d). The percentage of CCR5-expressing CD4+ T cells was significantly diminished in comparison with the value observed at baseline (P < 0.009). Similarly, the expression of CCR5 was significantly diminished on CD8+ T cells (P < 0.004) (Table 1). It should be noted that patients 16 and 17 were not included in the statistical evaluation because of scant compliance with the therapeutic regimen. The first patient suspended therapy within a few days, and the second took the drugs irregularly. For these reasons, all laboratory evaluations regarding these patients were considered separately from those of the other patients described in Fig. 1a–d. Interestingly, the decrease of CCR5 expression on CD4+ cells was not observed in the two patients (nos 16 and 17) who did not show any consistent decrease of viral load (Table 2).
Fig. 1.
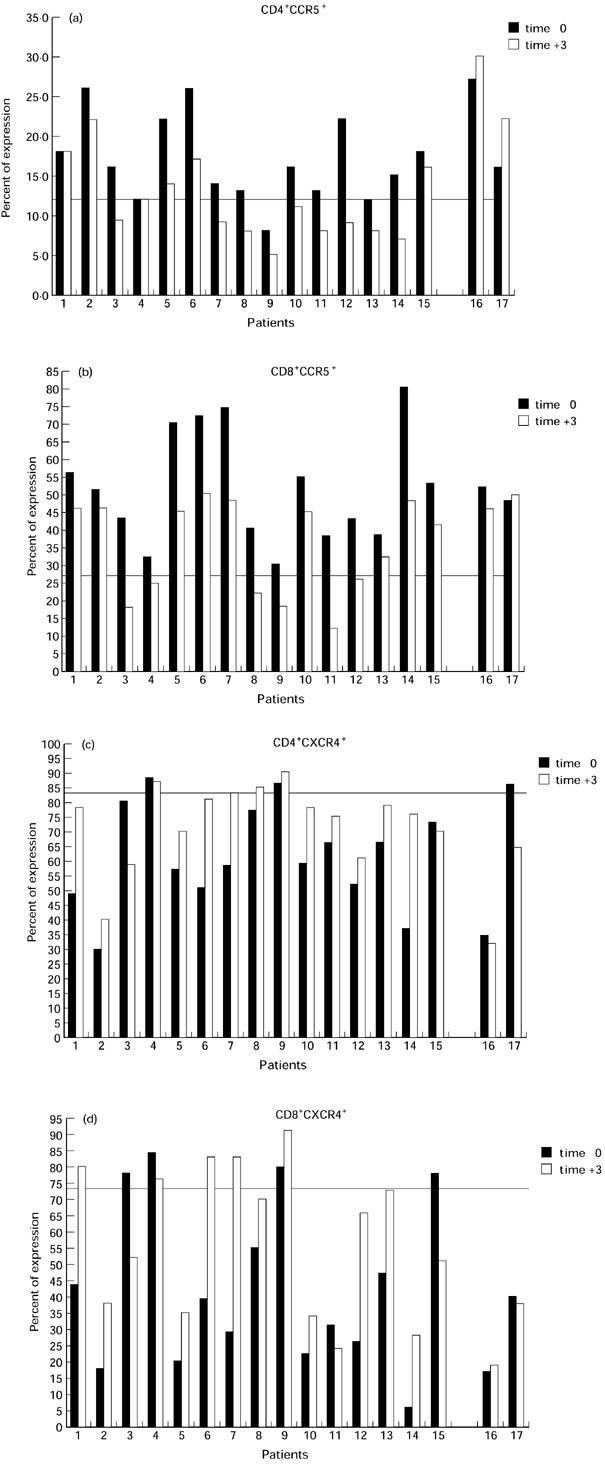
Reciprocal modifications of CCR5 and CXCR4 chemokine receptor expression on CD4+ and CD8+ T cells during early peripheral repopulation induced by highly active anti-retroviral therapy (HAART). Four-colour flow cytometric analysis was performed with peripheral blood mononuclear cells (PBMC) from HIV-1-infected individuals or normal blood donors at enrolment (time 0) and 3 months after HAART (time 3). The following MoAbs were used: anti-CD4–allophycocyanin (APC), anti-CD8–peridinin chlorophyll protein (PerCP), anti-CCR5–PE clone 2D7 and anti-CXCR4–PE clone 12G5. The expression of CCR5 before therapy was significantly increased in both CD4+ (a) and CD8+ T cells (b), while the expression of CXCR4 in the same cell types was down-regulated (c and d, respectively) compared with controls (represented by a line on graphs). After 3 months of HAART, the percentage of CCR5-expressing CD4+ (a) and CD8+ T cells (b) was significantly diminished, while CXCR4 expression was consistently increased (c and d, respectively).
Table 1.
CD4+ and CD8+ T cell subsets, naive cells (CD45RA+CD62L+), CCR5 and CXCR4 chemokine receptors and activation markers at time 0 and after 3 months of highly active anti-retroviral therapy (HAART)
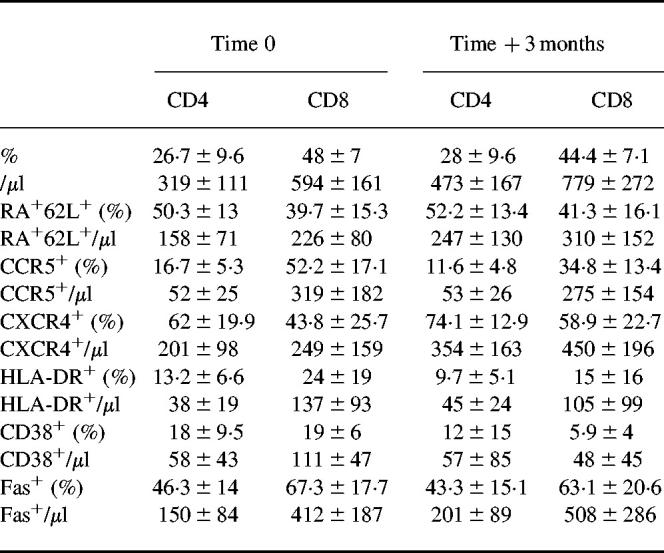
Table 2.
Plasma HIV RNA levels before and after 3 months of highly active anti-retroviral therapy (HAART)
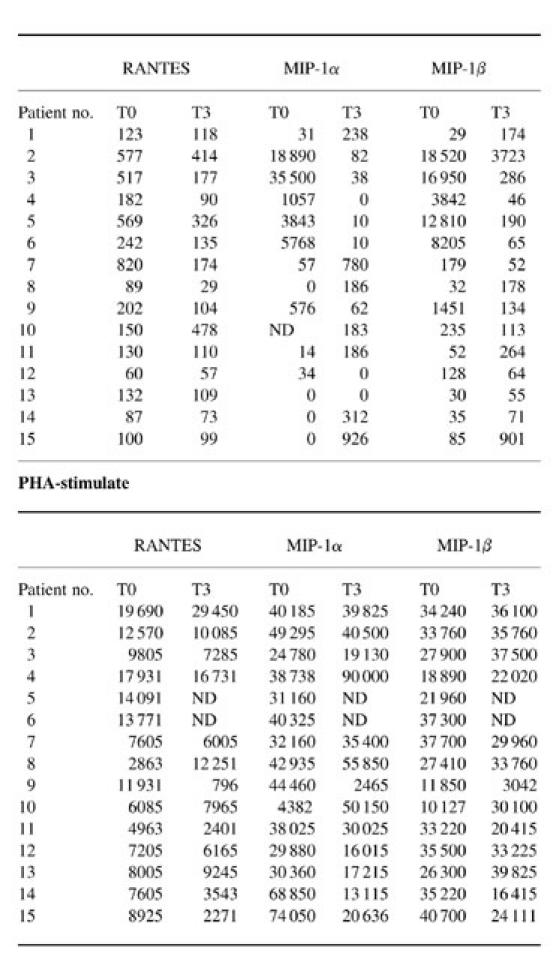
UND, Undetectable.
In contrast, as shown in Fig. 1c, CXCR4 expression was significantly increased after 3 months of therapy in CD4+ T cells (P = 0.036) but not in CD8+ T cells (P = 0.1) (Fig. 1d). In patients 16 and 17, however, the level of CXCR4-expressing CD4+ T cells was not increased but was further diminished. Interestingly, minor changes in CCR5 and CXCR4 expression were observed in patient 4, although a good virological and immunological response was achieved (plasma viral RNA from 56 000 to 28 copies/ml and CD4 T cell count from 349 cells/μl to 534 cells/μl). A possible explanation relates to the consistently low levels of systemic immune activation, documented by DR expression (9% and 10% at entry and after therapy, respectively), observed in this individual, a 30-year-old heterosexual female. This may possibly concur with the apparent stability of both CCR5 and CXCR4 expression.
The peripheral redistribution of naive and memory T cells in peripheral blood has been investigated by evaluating the differential expression of CD45RA and CD62L molecules on both CD4 and CD8 T cells. In fact, according to recent evidence, evaluation of CD45RA expression is not capable of fully discriminating naive from memory T cells. In particular, it has been shown that memory T cells, expressing CD45RO, that fail to re-encounter the specific antigen may revert to a CD45RA+ phenotype [23,24]. However, the expression of CD62L as well as that of CD44 remain down-regulated in such ‘revertants’, thus allowing a more detailed determination of naive T cells [25,26]. Results of these studies indicate a correlation between the levels of CXCR4 expression and the increment of both the absolute count and the percentage of CD4+/CD45RA+/CD62L+ naive T cells (r = 0.9, P < 0.0001 and r = 0.43, P = 0.011, respectively). An inverse correlation, although with a borderline significance, was also observed between CXCR4 expression and the decreased expression of T cell activation markers such as DR (r = 0.44, P = 0.009). On the other hand, the decreased expression of CCR5 was inversely related to the levels of CD45RA+/CD62L+ naive T cells in peripheral blood (r = 0.6; P = 0.0001). No significant correlation was observed between CCR5 expression and total CD4+ cell counts or DR+ cells, although the results show a trend similar to that observed for CXCR4. However, in CD4+ cells the expression of CCR5 was significantly related to that of CXCR4 (r = 0.75, P < 0.0001). It should be noted that the expression of both CXCR4 and CCR5 has been not evaluated in direct combination with the naive/memory phenotype. Therefore, the relationship between co-receptor expression and CD4+/CD45RA+/CD62L+ naive T cells just described derives from mathematical analysis and thus represents only a statistical correlation.
Measurements of spontaneous and lectin-induced chemokine production
Production of RANTES, MIP-1α and MIP-1β has been analysed in both the absence and presence of activation stimuli. Such an experimental setting allows the determination of the levels both of chemokines spontaneously produced by PBMC, which may more closely reflect their in vivo levels of production in tissue microenvironment, and of the activation-induced chemokines, which represent a potential for their production as a consequence of activation stimuli. The results are detailed in Table 3 and summarized in Fig. 2a,b. At enrolment, PBMC from HIV-1-infected individuals can spontaneously produce higher levels of β-chemokines compared with normal uninfected controls (Fig. 2a). However, a statistically significant difference was observed only with RANTES (P = 0.004), but not with MIP-1α and MIP-1β production (P = 0.27 and P = 0.12, respectively) (Fig. 2a). In fact, a detailed analysis of β-chemokine production in single patients (Table 3) revealed that the increments differed among HIV-1-infected individuals, though no relationship was found with either CD4+ cell count or plasma viral load at enrolment. Nevertheless, the levels of spontaneous β-chemokine production progressively decreased during HAART, reaching values closer to those detected with normal, uninfected controls (Fig. 2a). In particular, RANTES production at time 3 decreased in 14 out of 15 patients (Table 3) at levels not significantly different from that detected with normal controls (P = 0.09) (Fig. 2). In addition, the remaining individual (patient 10), who showed an increase of RANTES at time 3 (Table 3), revealed a delayed, though consistent decrease of RANTES production after 6 months of HAART regimen (from 478 pg/ml to 188 pg/ml). Similarly, lectin-induced synthesis of RANTES and MIP-1α was significantly higher in the group of HIV-1-infected individuals compared with normal controls (P = 0.002 and P = 0.05, respectively), while the difference in MIP-1β production was not statistically significant (P = 0.007) (Fig. 2b and Table 3). Again, differing increments were observed among HIV-1-infected individuals, though no relationship was found with either CD4+ cell count or plasma viral load at enrolment. However, after 3 months of HAART, lectin-induced RANTES and MIP-1α production decreased to levels consistent with those detected in control individuals (P = 0.09 and P = 0.25, respectively) (Fig. 2b). In contrast, MIP-1β production with PHA-stimulated cells was less consistently modified (Fig. 2b and Table 3), at least within the first 3 months of the HAART regimen.
Table 3.
Spontaneous and lectin-induced β-chemokine production by peripheral blood mononuclear cells (PBMC) from HIV-1-infected individuals
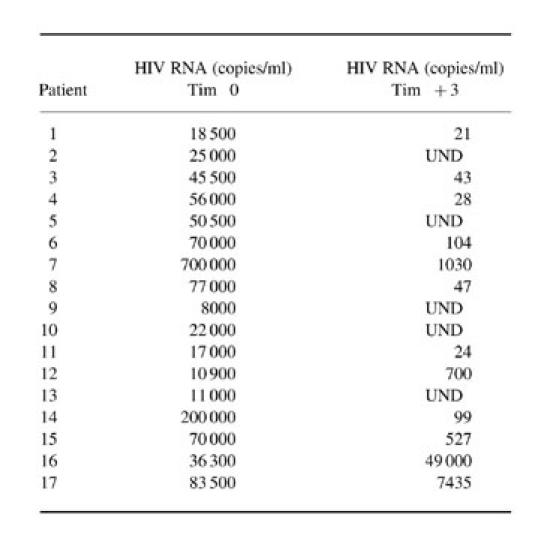
Unstimulated and phytohaemagglutinin (PHA)-induced RANTES, macrophage inflammatory protein 1α (MIP-1α) and MIP-1β production was determined by ELISA in supernatants from PBMC short-term cultures performed with freshly collected blood samples from HIV-1-infected individuals at enrolment (time 0) and 3 months after highly active retroviral therapy (HAART) (time 3). Results are expressed as pg/ml.
Fig. 2.
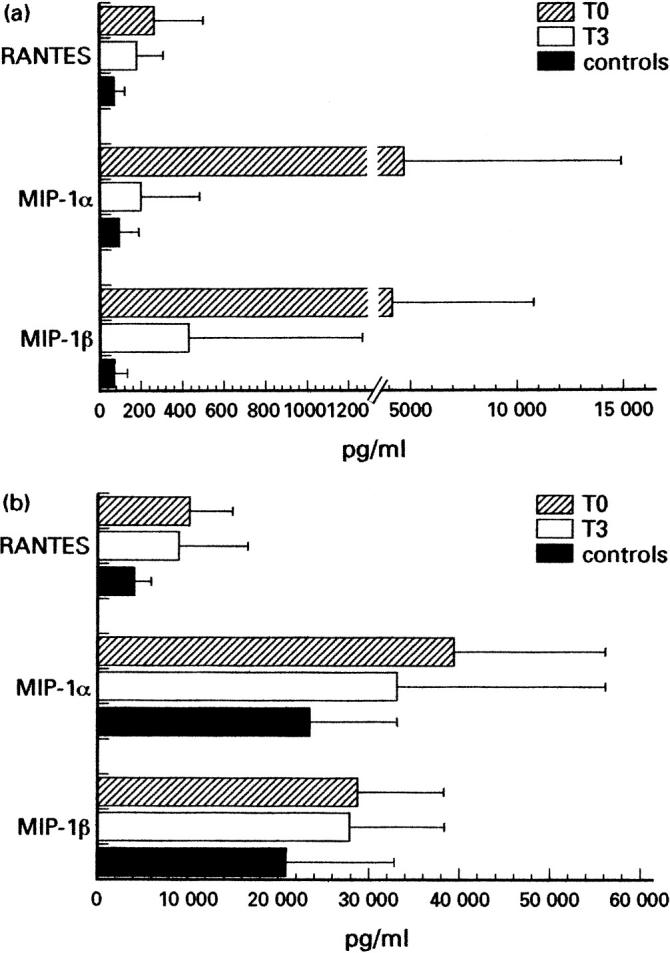
Modifications of spontaneous and lectin-induced β-chemokine production by peripheral blood mononuclear cells (PBMC) from HIV-1-infected individuals at enrolment (time 0) and 3 months after highly active anti-retroviral therapy (HAART) (time 3). Unstimulated (a) and phytohaemagglutinin (PHA)-induced (b) RANTES, macrophage inflammatory protein-1α (MIP-1α) and MIP-1β production was determined by ELISA in supernatants from PBMC short-term cultures performed with freshly collected blood samples from HIV-1-infected individuals or normal donors (controls). At enrolment, HIV-1-infected individuals produced higher levels of RANTES, MIP-1α and MIP-1β both spontaneously (a) and upon lectin-induction (b), compared with normal controls. However, both spontaneous and induced production of RANTES and MIP-1α consistently decreased after 3 months of HAART, reaching values closer to those detected in control individuals (a,b). MIP-1β lectin-induced production remained at the levels detected at enrolment (a,b).
DISCUSSION
T lymphocytes are capable of expressing most of the known CXC and CC chemokine receptors, their regulation depending upon the state of cell differentiation as well as the functional recruitment of the cells by activating stimuli [10,12]. In particular, the expression of two chemokine receptors, namely CXCR4 and CCR5, together with the regulated production of their respective ligands, appears to be extremely important in determining the susceptibility of T cells to HIV-1 infection [3,4,9,11,13,27].
In this study we have evaluated the levels of CCR5 and CXCR4 expression in HIV-1-infected individuals before and after HAART in order to understand more fully the relationship between virus replication and the expression of chemokine receptors which may support viral entry into target cells. In addition, we examined the production of β-chemokines, the ligands for the CC family of these receptors, in the same individuals. In fact, β-chemokines are produced by several cell types and in addition to acting as chemoattractants they may directly suppress the entry of macrophage-tropic strains in target cells [28].
We found an increased expression of CCR5 in PBMC from HIV-1-infected individuals compared with normal controls. Such increment was weakly related to DR expression on CD4+ T cells. A correlation was observed between CCR5 expression and the levels of CD45RA+/CD62L+ naive CD4+ T cells at enrolment. However, CCR5 expression was diminished after 3 months of HAART, and this was seen in concert with a consistent decrease of viral load as well as of the expression of immune activation markers and in association with the increased number of CD45RA+/CD62L+ naive T cells in peripheral blood. Among the CD4+ T cells, a significant correlation was observed between CCR5 expression and the percentage of both CD45RA+/CD62L+ naive and Fas-expressing cells. This evidence is reinforced by the observation that the two patients that demonstrated scarce compliance and a negligible virological response to HAART did not show such modifications of receptor expression. On the other hand, the levels of CXCR4 expression, which appear consistently down-regulated in both CD4+ and CD8+ T cells from HIV-1-infected individuals compared with uninfected controls, were significantly increased after 3 months of HAART. Within CD4+ cells the higher expression of CXCR4 was only weakly associated with the diminished expression of DR, while an inverse correlation was observed between CXCR4 and CCR5.
Since HAART results in a broad inhibition of systemic immune activation [29], such normalization of CCR5 and CXCR4 expression might be explained as a simple consequence of the reduced levels of immune activation. However, our data showing a lack of significant correlation between the levels of CXCR4-expressing cells and the expression of activation markers may also suggest an alternative hypothesis, at least for this receptor.
At present, the possibility of direct effects specifically induced by HIV-1 on CCR5 and CXCR4 expression in infected cells remains to be determined. However, similar modifications of chemokine receptors have been observed with other infectious diseases such as hepatitis C and infectious mononucleosis [13], suggesting that these alterations may represent a common feature of chronic virus-induced diseases, and do not specifically depend upon HIV-1 infection. In addition, we observed rebounded levels of both CCR5 and CXCR4 in a small subgroup of patients reaching 6 months of therapy (data not shown). This was seen in association with undetectable levels of plasma viraemia, minimal immune activation and with a marked rise of memory T cell counts in peripheral blood. Taken together, our data strongly suggest that the modifications of CCR5 and CXCR4 expression in peripheral blood may preferentially reflect the relative frequencies of naive versus memory T cells.
The β-chemokines RANTES, MIP-1α and MIP-1β, in addition to mediating immune cell recruitment and migration in target tissues, are known to interfere with the mechanism of entry of macrophage-tropic HIV-1 strains in target cells [28]. Thus, the pattern of chemokine production may contribute to suppressing viral transmission to neighbouring cells, but may also exert selective effects on the virus population. The results of the present study indicate that at enrolment, both the spontaneous and the PHA-induced production of β-chemokines by PBMC were higher in HIV-1-infected individuals compared with normal controls, although statistically significant differences were observed only with RANTES and MIP-1α. However, spontaneous production of RANTES, MIP-1α and MIP-1β decreased during HAART at levels closer to those determined in control individuals. Similarly, lectin-induced production of RANTES and MIP-1α decreased during HAART to levels consistent with those determined in normal individuals, while MIP-1β production remained higher than that observed with uninfected controls. These data indicate that the production of β-chemokines is up-regulated in HIV-1-infected individuals and suggest that their modification during HAART may depend upon the inhibition of viral replication and the decrease in levels of T cell activation. Results with lectin-activated PBMC also suggest that the higher potential for β-chemokine production upon immune-activating stimuli, which is expressed by these individuals, can be consistently reduced by HAART even in the early phases of the therapeutic regimen. These data indicate that an effective combined anti-retroviral therapy is capable of supporting a progressive ‘normalization’ of the production of inflammatory mediators which can act in controlling cell migration and invasion and may potentially contribute to triggering either direct and indirect tissue alterations [30]. At present, we do not know how lymphoid tissues play a part in such variations. However, although the decreased systemic immune activation may induce a relatively quiescent metabolic state in target cells which may further interfere with efficient viral replication, the peripheral modifications in the expression of host determinants of viral tropism and the reduced spontaneous production of anti-viral molecules may potentially contribute to supporting the emergence of virus variants in case of scarce compliance or interruption of therapy. This notion further emphasizes the importance of maintaining a highly effective viral suppression during HAART.
Acknowledgments
This work was supported by Grant no. 40A0.02, 1998 Istituto Superiore di Sanita’, recipient F.A.
REFERENCES
- 1.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC- CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 2.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 3.Choe H, Farzan M, Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 4.Carroll RG, Riley JL, Levine BL, Blair PJ, St LD, June CH. The role of co-stimulation in regulation of chemokine receptor expression and HIV-1 infection in primary T lymphocytes. Semin Immunol. 1998;10:195–202. doi: 10.1006/smim.1998.0131. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz ME, Cicala C, Arthos J, et al. Peripheral blood-derived CD34+ progenitor cells: CXC chemokine receptor 4 and CC chemokine receptor 5 expression and infection by HIV. J Immunol. 1998;161:4169–76. [PubMed] [Google Scholar]
- 7.Schuitemaker H, Koot M, Kootstra NA, et al. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–60. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu T, Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–81. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 9.Xiao L, Rudolph DL, Owen SM, Spira TJ, Lal RB. Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. Aids. 1998;12:F137–43. doi: 10.1097/00002030-199813000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–91. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mo H, Monard S, Pollack H, et al. Expression patterns of the HIV type 1 coreceptors CCR5 and CXCR4 on CD4+ T cells and monocytes from cord and adult blood. Aids Res Hum Retrovir. 1998;14:607–17. doi: 10.1089/aid.1998.14.607. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski MA, Justement SJ, Catanzaro A, et al. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998;161:3195–201. [PubMed] [Google Scholar]
- 14.Pakker NG, Notermans DW, de Boer BR, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 15.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 16.Pakker NG, Roos MT, van Leeuwen LR, et al. Patterns of T-cell repopulation, virus load reduction, and restoration of T-cell function in HIV-infected persons during therapy with different antiretroviral agents. J Acquir Immune Defic Syndr Hum Retrovir. 1997;16:318–26. doi: 10.1097/00042560-199712150-00002. [DOI] [PubMed] [Google Scholar]
- 17.Li TS, Tubiana R, Katlama C, Calvez V, Ait MH, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–6. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 18.Komanduri KV, Viswanathan MN, Wieder ED, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–6. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 19.Pandolfi F, Alario C, Girardi E, et al. The Italian quality control study for evaluation of CD4 cells in centres involved in the treatment of HIV-1 patients. Italian CD4 Quality Control Group. Clin Exp Immunol. 1998;111:564–73. doi: 10.1046/j.1365-2249.1998.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder JRR, Saget B, Scheibel S, Herman S, Payne H, Harrigan R, Kwok S. A rapid and simple method for extracting human immunodeficiency virus type 1 RNA from plasma: enhanced sensitivity. J Clin Microbiol. 1997;35:1278–80. doi: 10.1128/jcm.35.5.1278-1280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schockmel GA, Yerly S, Perrin L. Detection of low HIV-1 RNA levels in plasma. J Acquir Immune Defic Syndr Hum Retrovir. 1997;14:179–83. doi: 10.1097/00042560-199702010-00013. [DOI] [PubMed] [Google Scholar]
- 22.Fiorelli V, Gendelman R, Samaniego F, Markham PD, Ensoli B. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi's sarcoma spindle cells. J Clin Invest. 1995;95:1723–34. doi: 10.1172/JCI117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunce C, Bell EB. CD45RC isoforms define two types of CD4 memory T cells, one of which depends on persisting antigen. J Exp Med. 1997;185:767–76. doi: 10.1084/jem.185.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell EB, Sparshott SM, Bunce C. CD4+ T-cell memory, CD45R subsets and the persistence of antigen—a unifying concept. Immunol Today. 1998;19:60–64. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- 25.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Buck D, Terstappen LW. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–21. [PubMed] [Google Scholar]
- 26.Mackay CR. T-cell memory: the connection between function, phenotype and migration pathways. Immunol Today. 1991;12:189–92. 184–192. doi: 10.1016/0167-5699(91)90051-T. [DOI] [PubMed] [Google Scholar]
- 27.Bermejo M, Martin SJ, Oberlin E, et al. Activation of blood T lymphocytes down-regulates CXCR4 expression and interferes with propagation of X4 HIV strains. Eur J Immunol. 1998;28:3192–204. doi: 10.1002/(SICI)1521-4141(199810)28:10<3192::AID-IMMU3192>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Cocchi F, DeVico AL, Garzino DA, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 29.Andersson J, Fehniger TE, Patterson BK, et al. Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. Aids. 1998;12:F123–9. doi: 10.1097/00002030-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]


