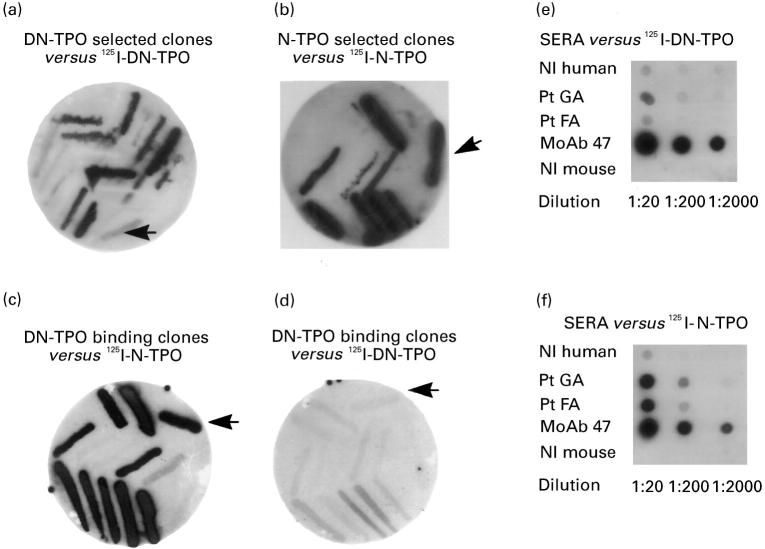
Fig. 1.
Interaction of thyroid-derived clones with native (N) versus denatured (DN) thyroid peroxidase (TPO) after three rounds of panning a pComb3H phage display library constructed from thyroid tissue of a patient with autoimmune thyroid disease. Individual clones from the third round of panning against DN-TPO or N-TPO were tested in filter lifts for binding of radiolabelled antigen. As anticipated, DN-TPO-selected clones bind 125I-DN-TPO (a) and N-TPO-selected clones bind 125I-N-TPO (b). Unexpectedly, DN-TPO binding clones interact better with 125I-N-TPO (c) than with 125I-DN-TPO (d). 125I-DN-TPO used in these studies is recognized very well by mouse MoAb 47, but only weakly or not at all by serum autoantibodies (patients GA and FA) (e). In contrast, 125I-N-TPO is recognized by both serum antibodies and mouse MoAb 47 (f). Controls include normal (Nl) human serum and normal mouse serum (e,f). The arrows indicate binding by the human monoclonal TPO autoantibody Fab WR1.7 (as a bacterial streak) that preferentially recognizes N-TPO [16].