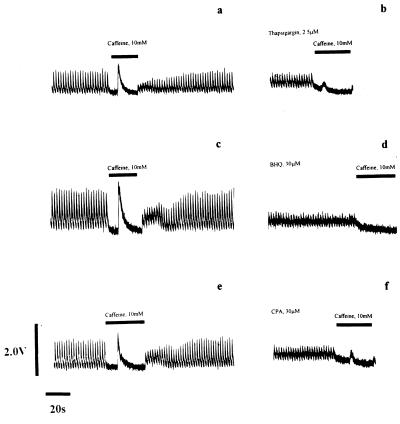
Figure 1.
Depletion of Ca2+ from the SR of rat ventricular myocytes by exposure to Ca2+-pump inhibitors. The intracellular [Ca2+] of individual rat ventricular myocytes (11) was measured by Fura-2 fluorescence ratio before (a, c, and e) and during (b, d, and f) exposure to Ca2+-pump inhibitors. Electrical stimulation of control cells in physiological saline solution generated Ca2+-transients of regular amplitude and short duration. The cessation of electrical stimulation and application of 10 mM caffeine (▪) provoked release of Ca2+ from the SR detected by a large rise in the Fura-2 fluorescence ratio (a, c, and e). Removal of caffeine and resumption of electrical stimulation facilitated refilling of the SR and recovery of the peak amplitude of the Ca2+-transient to control levels. Exposure of the cells to a Ca2+-pump inhibitor for 15 min [b, 2.5 μM Tg; d, 30 μM t-BHQ (BHQ); f, 50 μM CPA] caused loss of Ca2+ from the SR that resulted in a reduction in the Ca2+-transient size observed upon electrical stimulation, and ablation or massive attenuation of the Ca2+-transient induced by caffeine challenge (▪). A small rise in diastolic Ca2+ accompanied exposure to these drugs. Each row of the figure shows Fura-2 fluorescence before and after 15-min exposure to the SR Ca2+-pump inhibitor in the same cell.