Abstract
HTLV-1 has been implicated in certain pulmonary diseases. We previously demonstrated that expression of HTLV-1 tax/rex mRNA, encoding the transcriptional transactivator Tax, was closely associated with infiltration of activated T lymphocytes into lung tissue. To explore mechanisms of tax/rex expression in the lung, tax/rex mRNA expression and proviral DNA load were compared between peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage cells (BALC) from four patients with HTLV-1-associated myelopathy (HAM/TSP) and 13 carriers with various pulmonary symptoms. Semiquantitative detection of tax/rex mRNA strongly suggested that the lung was a preferential site for its expression. Proviral DNA loads in non-HAM/TSP carriers were variable but correlated well between PBMC and BALC in each individual, and revealed no relationship with tax/rex mRNA expression. In contrast, both cell groups from four HAM/TSP patients expressed detectable tax/rex mRNA accompanied by high proviral DNA load. The ratio of tax/rex mRNA expression to proviral DNA load was higher in BALC than in PBMC in three of four carriers and in three of four HAM/TSP patients, suggesting up-regulation of tax/rex mRNA in infected lung tissue. To analyse differences in distribution of HTLV-1 quasispecies between the two tissues, phylogenetic analysis was performed for sequence sets of the proviral tax open reading frame (ORF: 1059 bp) derived from PBMC and BALC of two infected individuals. Sequences derived from the two tissues distributed similarly to branches of phylogenetic trees, and there was no evidence of selective distribution of certain quasispecies in the lung. Our results suggest the presence of tissue-specific conditions that activate viral expression in infected cells in the lung. Constitutive exposure of this tissue to foreign antigens leading to up-regulation of basal viral promoter activity is likely to be one such mechanism.
Keywords: HTLV-1, tax/rex mRNA, bronchoalveolar lavage, quasispecies, local availability
Introduction
HTLV-1 is a retrovirus involved in the pathogenesis of adult T cell leukaemia (ATL) and non-neoplastic inflammatory diseases of various organs. The latter include HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), HTLV-1-associated uveitis (HAU), rheumatoid arthritis, Sjögren's syndrome and pneumonopathy [1]. With respect to pulmonary involvement in HTLV-1 infection, patients with HAM/TSP and HAU frequently exhibit pulmonary complications characterized by T-lymphocytic alveolitis or lymphocytic interstitial pneumonia [2–4]. Neither leukaemic cells nor pathogens associated with opportunistic infections are found in the lungs of these patients. Similar pulmonary involvement has been reported in asymptomatic carriers of HTLV-1 [5]. Although pulmonary involvement in patients with HAM/TSP and HAU is usually subclinical, T cell lymphocytic alveolitis can be demonstrated by bronchoalveolar lavage (BAL) in 60–80% of cases [6]. A characteristic pathological feature is infiltration of mononuclear cells in submucosal tissue of bronchioles, perivascular areas of the parenchyma, and alveolar septa [7]. The infiltrating cells are predominantly CD3+ T lymphocytes.
Pathogenic mechanisms of HTLV-1 in lung tissue are not fully elucidated. Cultured HTLV-1-infected T cells are known to exhibit activated T cell phenotypes and produce various cytokines and their receptors [8,9]. HTLV-1-infected cells in lung may locally induce T cell expansion and chronic inflammation via cognate cell–cell interaction with non-infected T cells and/or production of cytokines and their receptors [10]. Consistent with this is the evidence that cultured pulmonary and peripheral blood T lymphocytes from HAM/TSP patients spontaneously proliferate and release IL-2 into the culture medium [11]. In addition, increased IL-2 receptor α (IL-2Rα: CD25)-bearing T cells and high levels of soluble IL-2R were noted in BAL cells (BALC) and BAL fluid (BALF), respectively, from HAM/TSP patients with T-lymphocytic alveolitis [7,12]. Involvement of the cytotoxic immune response against viral antigens in the lung has also been suggested [6].
We previously reported that detection of tax/rex mRNA, the doubly spliced transcript of HTLV-1, correlated closely with the presence of lymphocytosis and increased proportion of IL-2R-bearing T cells in BALC [13]. tax/rex mRNA encodes a transcriptional transactivator, Tax, which up-regulates the viral promoter and also expression of various cellular genes including IL-2, IL-2Rα, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and c-fos [1,8,9]. Moreover, Tax is thought to be the major target molecule of the cytotoxic T cell response against HTLV-1 in vivo [14,15]. It is conceivable that Tax plays a crucial role in the inflammatory response in the lungs of HTLV-1-infected individuals.
The present study was designed to explore mechanisms of tax/rex mRNA expression in lung tissue. For this purpose, we compared the level of tax/rex mRNA expression, proviral DNA load, and HTLV-1 quasispecies between peripheral blood mononuclear cells (PBMC) and BALC obtained from 17 infected individuals. Our results suggest involvement of tissue-specific mechanisms, which activate viral expression in infected cells in the lung.
PATIENTS and METHODS
Patients
We examined 17 seropositive individuals (four males and 13 females) ranging in age from 16 to 74 years. Seropositivity was determined using both the particle agglutination (Serodia-HTLV-1; Fuji Rebio, Tokyo, Japan) and indirect immunofluorescence tests, and was confirmed by immunoblotting. The patients comprised four with HAM/TSP and 13 non-HAM/TSP patients, designated as carriers. The latter group included three patients with sarcoidosis, one with pulmonary tuberculosis, and two with pneumonia due to bacterial infection, and the remaining individuals complained of mild cough and sputum. There were no cases of viral infection other than HTLV-1 or autoimmune diseases. Informed consent was obtained from all patients prior to the commencement of the study, and the human experimentation guidelines of Nagasaki University were followed in conducting clinical research.
Cell line
We used the HTLV-1-infected human cell line MT-2 as a control [16].
Preparation and analysis of cells
BAL was performed under local anaesthesia. The fibrescope was inserted into the right middle lobe and 150 or 200 ml of sterile saline solution in three or four aliquots of 50 ml were instilled. The fluid was recovered by gentle aspiration into a sterile syringe and transported to the laboratory at 4°C within 30–60 min. Lavage fluid was passed through two sheets of gauze and BALC were pelleted by centrifugation at 400 g for 10 min at 4°C. PBMC were separated from heparinized venous blood by Ficoll–Hypaque gradient centrifugation. Aliquots of the cell suspension were prepared for immunocytochemical staining and DNA/RNA harvest. An aliquot of the suspension was subjected to flow cytometry (FACScan; Becton Dickinson, Mountain View, CA) after staining with FITC-conjugated anti-CD3 MoAb (Becton Dickinson) to determine the proportion of CD3+ cells.
DNA and RNA preparation
Approximately 2 × 106 cells from PBMC and BALC were lysed in 0·5 ml lysis buffer (150 mm NaCl, 10 mm Tris–HCl pH 8·0, 10 mm EDTA, 0·5% SDS) and treated with proteinase K (100 μg/ml) for 2 h at 50°C. After phenol/chloroform extraction and ethanol precipitation, high molecular weight DNA was resuspended in 10 mm Tris (pH 8·0)/1 mm EDTA. Total RNA was isolated by TRIzol reagent (Gibco BRL, Rockville, MD) according to the protocol recommended by the manufacturer.
Semiquantitative detection of tax/rex mRNA by reverse transcription-polymerase chain reaction
RNA samples (adjusted to 1 μg/10 μl) were serially diluted 10-fold with a solution containing yeast tRNA (1 μg/10 μl). Diluted samples were subjected to reverse transcriptase-polymerase chain reaction (RT-PCR) targeting the doubly spliced tax/rex mRNA. Reverse transcription and PCR were performed as previously described [13]. Briefly, cDNA was synthesized using the sequence-specific primer oligonucleotide pX9 and Superscript II Reverse Transcriptase (Gibco BRL). The cDNA was then subjected to successive two-step amplification using nested primer pairs of the second splice junction site of tax/rex mRNA [13,17]: step 1, forward primer (RPX-11): 5′-TAATAGCCGCCAGTGGAAAG, reverse primer (pX-9): 5′-TGATCTGATGCTCTGGACAG; step 2, forward primer (RPX 3): 5′-ATCCCGTGGAGACTCCTCAA, reverse primer (RPX-4): AACACGTAGACTGGGTATCC, respectively. The amplified product (145 bp) was analysed on a composite gel containing 2% NuSieve/1% SeaKem agarose (FMC, Rockland, ME) stained with ethidium bromide. Expression of tax/rex mRNA was estimated by the end-point dilution which gave the expected band on the gel. Experiments were performed twice to confirm the quantification.
Determination of proviral DNA load by nested PCR
Nested oligonucleotide primer pairs were designed to selectively amplify the 119-bp fragment of HTLV-1 pX gene in a two-step process: step 1, forward primer (pX-1): 5′-CCCACTTCCCAGGGTTTGGACAGAG, reverse primer (pX-2): 5′-CTGTAGAGCTGAGCCGATAACGCG; step 2, forward primer (pX 8): 5′-ACCCAGTCTACGTGTTTGGA, reverse primer (pX-9): TGATCTGATGCTCTGGACAG, respectively. PCR was performed in a TRIO-Thermal Cycler TB-1 (Biometra, Göttingen, Germany) for 30 cycles as previously described [18]. Briefly, the mixture including 0·5 μg sample DNA was denatured at 94°C for 2 min, annealed at 60°C for 2 min and extended at 72°C for 2 min. For nested PCR, 1/10 volume of the first-step PCR product purified by SuperRecII column (TaKaRa Co., Tokyo, Japan) was further amplified with the same thermal cycle programme for 27 cycles, using the inner primer pair. This nested PCR has been shown to be sensitive enough to detect a single molecule of the HTLV-1 provirus [18]. For the purposes of semiquantitative detection, DNA samples were serially diluted 10-fold and two-fold successively, and each dilution was then subjected to nested PCR. The proviral DNA load was estimated from the end-point dilution which gave an amplified band in an agarose gel, on the basis that 0·5 μg DNA was equivalent to that obtained from 1 × 105 cells. Experiments were performed twice to confirm the quantification.
Amplification, subcloning, and sequencing of the tax open reading frame of HTLV-1 proviruses
The entire tax open reading frame (ORF) of the HTLV-1 provirus was amplified by nested PCR. The primers used were: Tax11 (5′-GATAGCAAACCGTCAAGCACAG-3′; position 7158–7179) and Tax6 (5′-TCCTGAACTGTCTCCACGCAAA-3′; 8650–8629) for the first amplification, and Tax15Xba (5′-TACTCTAGAACCATGGCCCACTTCCCAGG-3′; 7324–7337) and Tax30Bam (5′- CTGAGGATCCAGAGCCTTAGTCT-3′; 8409–8397) for the second amplification. Xb aI and Bam HI restriction sites (underlined) were included in Tax15Xba and Tax30Bam primers, respectively, to allow subsequent subcloning into the expression plasmid pCG [19]. PCR was performed in a TRIO Thermal Cycler TB-1 (Biometra) with the following conditions: 30 cycles of denaturation at 94°C for 30 s (2 min on the first cycle), annealing at 60°C for 30 s, and extension at 72°C for 70 s (7 min on the last cycle). For nested PCR, 1/10 volume of the first-step PCR product purified by SuperRecII column (TaKaRa Co.) was further amplified for 27 cycles using the inner primer pair. The thermal cycle programme was similar to that used for the first PCR, except the annealing temperature was 58°C for the first six cycles and 63°C for the following 21 cycles. The nested PCR product was purified on a 0·8% agarose gel (SeakemGTG; FMC), digested with Xba I and Bam HI, and then ligated into pCG. After transformation of Escherichia coli (DH5α), recombinant clones were randomly picked and plasmid DNA was miniprepped by the NaOH/SDS method. The entire tax ORF (1059 bp) in the plasmid DNA was sequenced using the dideoxy chain termination method (Thermo Sequence core sequencing kit; Amersham, Aylesbury, UK) with two 5′ Texas red-labelled primers corresponding to the flanking vector sequence (HSK-tk sequence, 5′-GCCCAGCGCCTTGTAGAA 3′; and rabbit-β-globin sequence, 5′-TAGGCGA AAAAGAAAGAAC-3′), and then analysed on an automated sequencer (SQ5500; Hitachi Co., Tokyo, Japan). A sequence comparison was carried out using ATK-1 sequence [20].
Phylogenetic analysis
The multiple alignment and the phylogenetic analysis for the nucleotide sequences of the tax ORF were conducted using the computer software CLUSTAL [21]. In practice, the phylogenetic trees were reconstructed using the neighbour-joining method [22] with the evolutionary distances estimated by the Kimura's two-parameter method [23]. The phylogenetic trees were visualized by TREEVIEW [24].
Statistical analysis
Data were expressed as mean ± s.d. Geometric analysis was used for log scale correlation. Statistical comparison was performed using Kruskal–Wallis rank test to examine differences between means of unpaired samples, and Spearman's rank correlation was used to examine the relationship between various parameters. Statistical analysis was performed using the Statview-J 4.5 software package. P < 0·05 was considered significant.
Results
The lung is a preferential site for tax/rex mRNA expression in HTLV-1-infected individuals
To compare tax/rex mRNA expression between the lung and peripheral blood, tax/rex mRNA levels in BALC and PBMC from four HAM/TSP and 13 non-HAM/TSP carriers were estimated by RT-PCR of serial 10-fold dilutions of each RNA sample. tax/rex mRNA was undetectable even in undiluted RNA samples from both tissues from six carriers. In the other three carriers it was detectable only in BALC but not in PBMC (Fig. 1). There was no individual in whom tax/rex mRNA was detectable in PBMC but not in BALC. tax/rex mRNA was amplifiable from both tissues from four HAM/TSP patients and the remaining four carriers. As shown in Fig. 1, in six of these eight individuals (carrier 3/4, and HAM/TSP 3/4), the amplifiable end-point of the BALC sample was 10–100 times weaker than that of the corresponding PBMC sample, indicating higher tax/rex mRNA expression in BALC. The remaining two individuals (carrier 1/4, and HAM/TSP 1/4) exhibited equivalent levels of expression in both tissues. These findings confirmed the lung tissue as a preferential site for tax/rex mRNA expression. Consistent with our previous report [13], the BALC samples with detectable tax/rex mRNA exhibited lymphocytosis with a significantly higher proportion of CD3+ cells (data not shown).
Fig. 1.
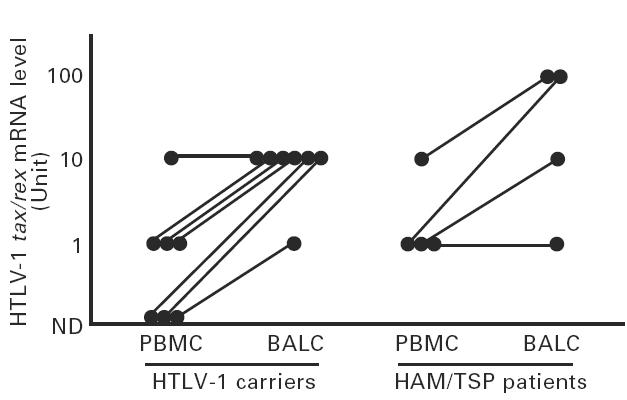
Comparison of tax/rex mRNA expression between peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage cells (BALC) from HTLV-1-infected individuals. Reciprocal values of the end-point dilution (Unit), which contained tax/rex mRNA amplifiable by reverse transcriptase-polymerase chain reaction (RT-PCR), of RNA samples from seven carriers and four HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients are plotted and compared between PBMC and BALC from each individual. ND, Not detected.
Proviral DNA loads do not correlate with tax/rex mRNA expression in BALC and PBMC of non-HAM/TSP carriers
Proviral DNA load is thought to be a crucial factor determining the expression level of tax/rex mRNA. To evaluate this, the proviral DNA load in each tissue sample was determined by use of nested PCR with the sensitivity to amplify the HTLV-1 pX sequence from 5 × 10−7μg DNA from an infected cell line, MT2, roughly equivalent to a single molecule of the HTLV-1 provirus (Fig. 2). Proviral DNA loads in PBMC and BALC of non-HAM/TSP carriers were variable, ranging from 100·0 to 103·0 and 100·0 to 103·3 copies/μg DNA, respectively. There was no correlation between proviral DNA loads and tax/rex mRNA expression. Geometric mean values of proviral DNA loads in PBMC and BALC samples in which tax/rex mRNA was detectable, 2·25 ± 0·50 (geometric mean[log10] ± s.d.) and 1·90 ± 1·27, respectively, were almost equivalent to those values of the samples without detectable tax/rex mRNA (1·99 ± 1·19 and 1·77 ± 1·00). In contrast, HAM/TSP patients exhibited high proviral DNA loads in both tissues, ranging from 103·0 to 104·6 copies/μg DNA (geometric mean ± s.d.: 3·65 ± 0·79) in PBMC and 103·0 to 105·2 copies/μg DNA (3·80 ± 1·05) in BALC, which were significantly higher than those of carriers' samples without tax/rex mRNA expression (P < 0·05, Fig. 3).
Fig. 2.
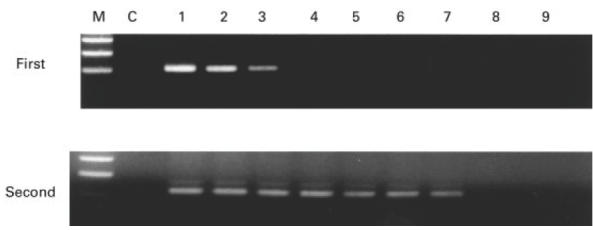
Amplification of HTLV-1 proviral DNA using nested polymerase chain reaction (PCR). One microgram of DNA from the HTLV-1-infected cell line, MT2, containing about 1·0 × 106 molecules of HTLV-1 provirus was diluted 10-fold (100−10−8), and each dilution was subjected to two-step PCR using nested primer pairs. PCR products from the first-step and second-step amplification were analysed on agarose gels. M, Molecular size markers; C, negative controls (amplification from 1 μg of HTLV-1-negative cell DNA).
Fig. 3.
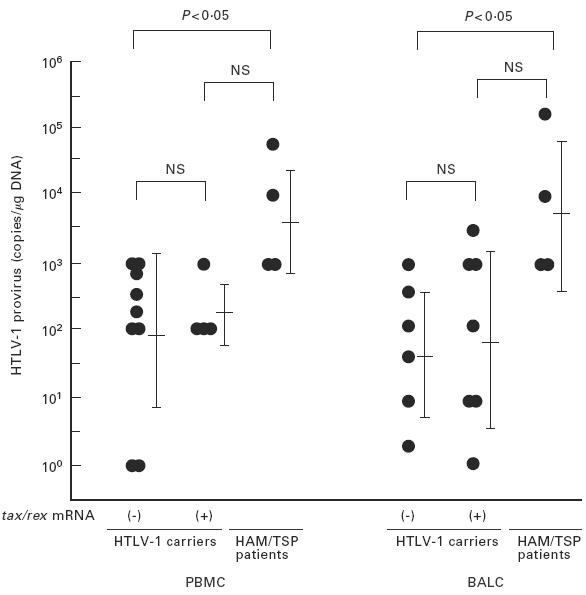
Lack of correlation between tax/rex mRNA expression and virus load in bronchoalveolar lavage cells (BALC) of non-HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) carriers. Virus load (copies/μg DNA) in peripheral blood mononuclear cells (PBMC) and BALC semiquantified by nested polymerase chain reaction (PCR) was compared between carriers (with and without tax/rex mRNA expression) and HAM/TSP patients. Virus loads in HAM/TSP patients were significantly (P < 0·05) higher than in carriers without detectable tax/rex mRNA, but the difference in values between the two carrier groups was not significant.
Correlation between proviral DNA load in PBMC and in BALC
Direct comparison of proviral DNA loads between PBMC and BALC in each individual is shown in Fig. 4a. Positive correlation was observed between proviral DNA loads in PBMC and BALC (r = 0·82, P < 0·01). Both tissues from HAM/TSP patients, which exclusively expressed detectable tax/rex mRNA, revealed high proviral DNA loads. While there was no evidence of preferential accumulation of infected cells in lung tissue of carriers, even in those that tax/rex mRNA was detectable only in BALC (Fig. 4a). According to the results of flow cytometric analysis, the proportion of T lymphocytes (CD3+ cells) in BALC was highly variable among individuals, ranging from 2·0% to 66·2% among BALC samples, in contrast to a small variation among PBMC samples (10·8–35·0%). The values for proviral DNA load were thus calibrated with the proportion (%) of CD3+ cells in each sample, and the resulting proviral DNA loads in the T cell population were compared between PBMC and BALC. As shown in Fig. 4b, this value was more closely correlated between PBMC and BALC (r = 0·90, P < 0·01). This indicated that the proportion of infected cells, particularly in the T cell population, was not different between the two tissues and argued against selective migration of infected T cells into lung tissue.
Fig. 4.
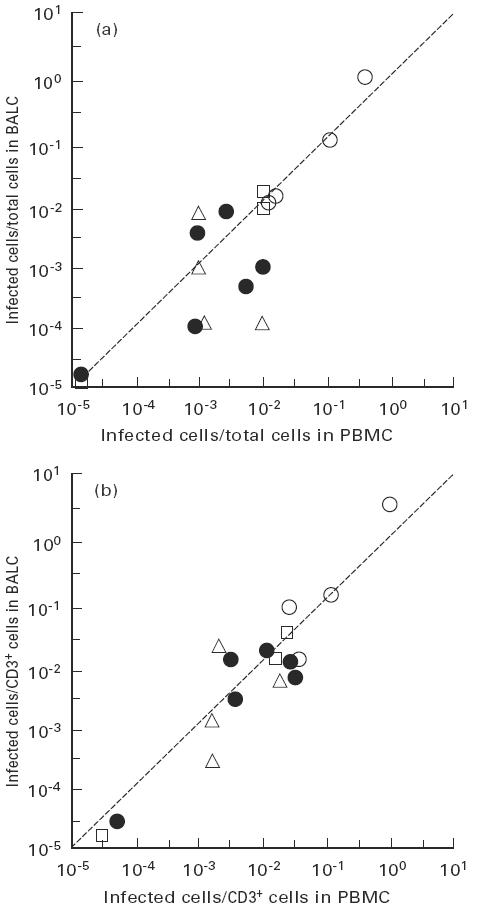
Correlation of virus load between peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage cells (BALC) of each infected individual. Viral loads in total cells (a) and in CD3+ T cells (b) were compared between PBMC and BALC of each individual. ○, HTLV-1-associated myelopathy/tropical spastic paraparesis patients; •, carriers without detectable tax/rex mRNA in either tissue; □, those with detectable tax/rex mRNA only in BALC; Δ, those in whom tax/rex mRNA was detectable in both tissues.
Transcription of tax/rex mRNA is activated in lung tissue
tax/rex mRNA expression in infected cells was compared between PBMC and BALC in each of eight individuals, including four patients with HAM/TSP, using values obtained by dividing tax/rex mRNA expression level in total cells (Units, shown in Fig. 1) by proviral DNA load. Three of four carriers exhibited 10–1000 times higher ratios in BALC compared with corresponding PBMC (Fig. 5), suggesting that tax/rex mRNA expression was up-regulated in infected cells in the lung. To a lesser extent but still significant, up-regulation of 2·5–100 times was observed in BALC, even in three of four HAM/TSP patients. To confirm this finding, DNA and RNA were freshly isolated from PBMC and BALC of two additional individuals, including one HAM/TSP patient, and virus load and tax/rex mRNA expression were analysed in parallel. One microgram each of DNA and RNA samples was serially five-fold diluted and the dilutions were then subjected to PCR and RT-PCR, respectively (Fig. 6). In case 1 (HAM/TSP), the end-point dilutions of DNA from PBMC and BALC with amplifiable HTLV-1 genome were 5−5 and 5−4, respectively, while tax/rex mRNA was detectable in RNA dilutions of 5−2 and 5−3, respectively. Case 2 (carrier) revealed a more striking difference, i.e. end-point dilutions in the two tissue samples were 5−5versus 5−2 (PBMC versus BALC) for DNA and 5−1versus 5−3 for RNA. The higher expression level of tax/rex mRNA despite lower proviral DNA load in BALC was consistent with preferential activation of HTLV-1 in the lung.
Fig. 5.
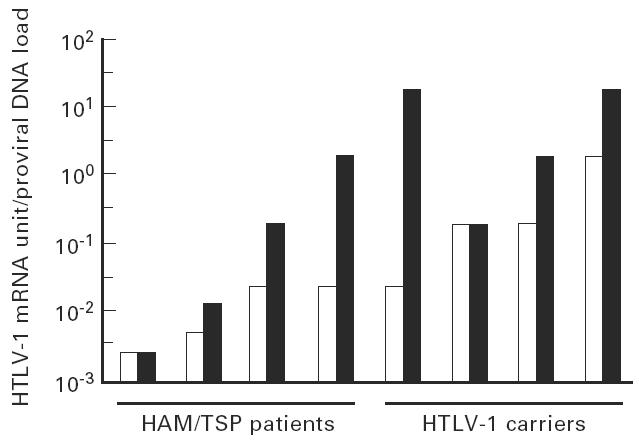
Levels of tax/rex mRNA in HTLV-1-infected cells were higher in bronchoalveolar lavage cells (BALC; ▪) than peripheral blood mononuclear cells (PBMC; □). Ratios between tax/rex mRNA levels (Units—shown in Fig. 1) and virus load were compared between PBMC and BALC of four carriers and four HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients who expressed tax/rex mRNA.
Fig. 6.
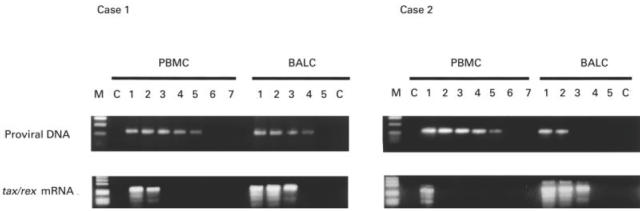
Semiquantitative comparison of tax/rex mRNA expression and virus load in peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage cells (BALC). Serial five-fold dilutions of DNA and RNA (50−5−6 μg DNA and RNA, respectively) from PBMC and BALC of two infected individuals (cases 1 and 2) were subjected to polymerase chain reaction (PCR) and reverse transcriptase (RT)-PCR, respectively. M, Molecular size markers; C, negative controls (amplification from 1 μg of HTLV-1-negative DNA for PCR and from 1 μg of sample RNA without reverse transcription for RT-PCR).
Similar distribution of HTLV-1 quasispecies in PBMC and BALC
To evaluate the possibility of selective migration of HTLV-1 quasispecies in the lung, variation in the HTLV-1 proviral DNA sequence corresponding to the tax ORF (1059 bp) derived from PBMC and BALC of a HAM/TSP patient and a healthy carrier was analysed. As shown in Fig. 7b, sequence comparison revealed two consensus sequences in the HAM/TSP patient. Among the 40 sequences (20 each from PBMC and BALC) analysed, 27 contained one to four nucleotide substitutions from the consensus sequences. Sequences derived from two consensuses were equivalently distributed in PBMC and BALC. All 40 sequences from the healthy carrier (20 each from PBMC and BALC) were derived from a distinct consensus sequence, and in 22 of these randomly localized nucleotide substitutions were found (Fig. 7c). The frequency of nucleotide substitutions in sequences similarly obtained from a cloned HTLV-1 plasmid, pMT2, was about six times less than that found among the sequences from individuals (Fig. 7a), indicating that our results represented HTLV-1 quasispecies in vivo. Phylogenetic trees constructed from the sequence sets of each individual are shown in Fig. 8a,b. Both sequences derived from PBMC and BALC were randomly distributed in three branches of the tree, corresponding to the consensus sequences. There was no evidence of selective distribution of certain HTLV-1 quasispecies in the lung.
Fig. 7.
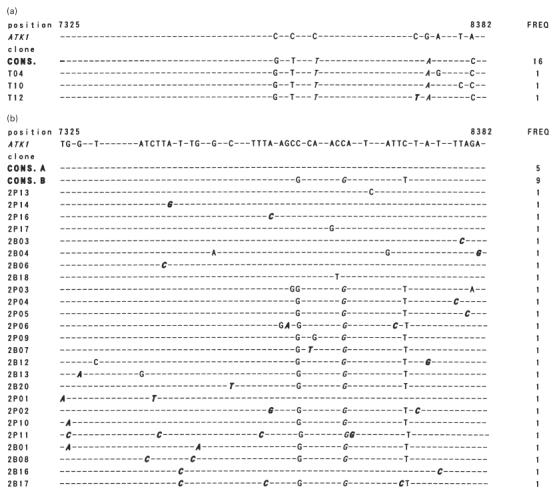
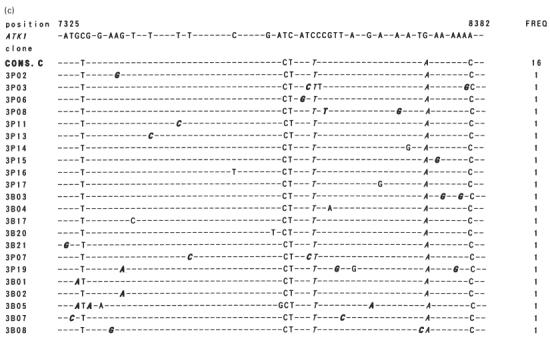
(See next page.) Nucleotide sequence variation among in vivo-derived HTLV-I tax clones. The whole Tax open reading frame (ORF) amplified from a sample DNA was ligated into pCG, and randomly selected plasmid clones were sequenced. Nucleotide sequences of 19, 39, and 38 tax clones derived from a cloned HTLV-I, pMT2 (a), and two HTLV-I-infected individuals, I.T. (b) and N.M. (c), respectively, are aligned in comparison with the reference ATK1 [20]. Dots indicate sequences identical to the reference. Italicized letters indicate non-synonymous substitutions, and those resulting in the translational terminal codon, in particular, are underlined. The dominant sequences in each sample are indicated as consensus sequences (CONS.). The number of clones identical to a given nucleotide sequence (FREQ) is shown on the right.
Fig. 8.
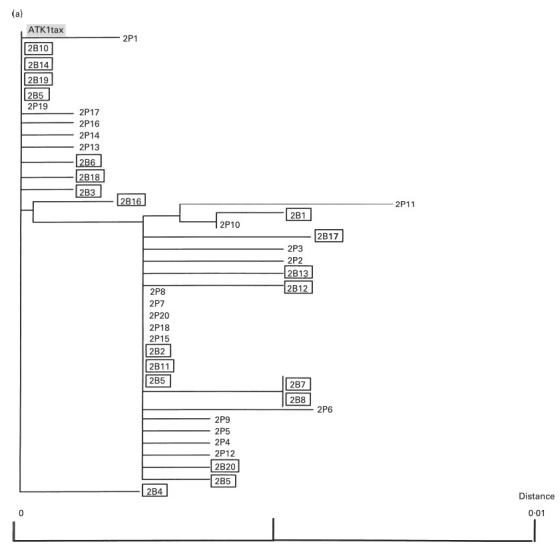
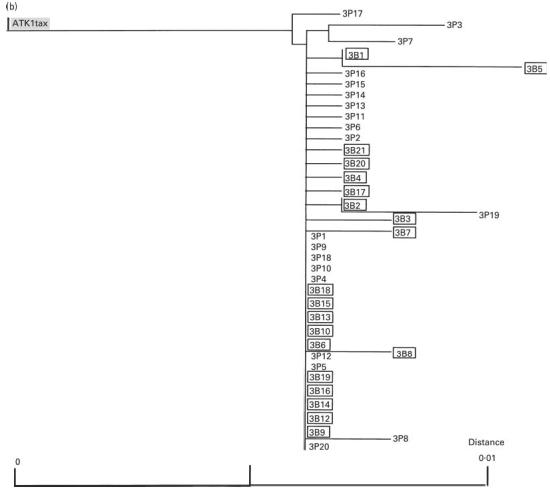
(See next page.) Phylogenetic trees for sets of tax open reading frame (ORF) sequences derived from peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage cells (BALC) from two infected individuals ((a) HTLV-1-associated myelopathy/tropical spastic paraparesis; (b) carrier). The neighbour-joining tree was created using the Kimura two-parameter method [23]. The letters P and B indicate the clones obtained from PBMC and BALC, respectively. Numbers represent the clone numbers. The clones derived from BALC are in squares. ATK-1 tax is a control sequence. The scale bars represent the percentage nucleotide sequence divergence.
Discussion
Semiquantitative detection of tax/rex mRNA strongly suggested that lung tissue is a preferential site for its expression. Of 17 infected individuals analysed, tax/rex mRNA was detectable in both or either PBMC and BALC in 11 individuals. It was detectable only in BALC but not in PBMC in three individuals. The remaining eight, including all four HAM/TSP patients, exhibited expression in both tissues, but expression levels were higher in BALC than PBMC in six of the eight. No patients exhibited preferential tax/rex mRNA expression in the PBMC. The high number of infected cells (high proviral DNA load), selective distribution of infected cells with an advantage for viral expression, and/or presence of tissue-specific conditions activating viral expression in infected cells were presumed to underlie preferential tax/rex mRNA expression in the lung. To evaluate these possibilities, we analysed proviral DNA loads and quasispecies in PBMC and BALC.
High-level viral expression together with extremely high proviral DNA load is well documented in PBMC from HAM/TSP patients. It is possible that tax/rex mRNA expression levels simply reflect proviral DNA load. Cultured HTLV-1-infected cells exhibit the activated T cell phenotype which includes expression of various cell adhesion molecules, such as CD2, CD45, and LFAs, on the cell surface [10]. Expression of these cell adhesion molecules is thought to give the cells an advantage to migrate and infiltrate into visceral organs, including the lung, and may result in the accumulation of infected cells in the organ. Consistent with previous findings [4,25], both tissue types from HAM/TSP patients in the present study showed exclusive tax/rex mRNA expression and high proviral DNA load, indicating the contribution of high proviral DNA load in tax/rex expression in HAM/TSP. In contrast, there was no correlation between proviral DNA load and detection of tax/rex mRNA in non-HAM/TSP patients. In particular, three BALC samples expressed detectable tax/rex mRNA despite extremely low proviral DNA load (< 10 copies/μg DNA; Fig. 3), indicating that proviral DNA load is not the factor causing higher viral expression in the lung. Moreover, proviral DNA load, in particular in the T cell population, was closely correlated between PBMC and BALC in each individual, including HAM/TSP patients. This clearly argues against the selective migration and accumulation of infected cells in lung tissue and suggests involvement of factors up-regulating viral expression in the tissue.
Direct comparison of the ratio between expression of tax/rex mRNA and proviral DNA load indicated that expression was preferentially up-regulated in infected lung cells. The difference in up-regulation between PBMC and BALC was more obvious in non-HAM/TSP carriers and less in HAM/TSP patients. Factor(s) that up-regulate viral expression in lung is likely to be more profoundly involved in carriers and increase tax/rex mRNA expression to detectable levels even in lungs with an extremely low proviral DNA load.
HTLV-1 is a very old virus which has been maintained in the population through sexual and vertical transmission [26,27]. Little divergence in the nucleotide sequence was identified among HTLV-1 isolates derived from different geographical regions of the world [28]. However, accumulating evidence has recently indicated the presence of intrastrain variability (quasispecies) of HTLV-1 [29–31]. It was demonstrated that amino acid substitutions in the gene encoding Tax frequently resulted in functional loss of the protein and a significant proportion of HTLV-1 quasispecies of PBMC in infected individuals encoded the defective Tax [32,33]. Since Tax function is indispensable for viral expression [34], such a quasispecies is not likely to be infectious. The quasispecies was therefore considered to be a potential factor involved in tissue-specific differences in viral expression. To evaluate the possibility that quasispecies competent for Tax function was selectively distributed in lung and that a particular subset of infected cells revealed tropism for the organ, we performed phylogenetic analysis for 80 sequence sets of tax ORF derived from PBMC and BALC of two infected individuals. The sequences from both tissues were equivalently distributed in branches of the phylogenetic tree, arguing against differences in quasispecies between tissues. Preliminary experiments to evaluate the function of Tax encoded by 80 sequences (Fig. 7) in cells co-transfected with a reporter plasmid indicated that 16 sequences (20·5%) that appeared to encode non-functional Tax were equivalently distributed in PBMC and BALC (data not shown). These findings suggest that the quasispecies is unlikely to contribute to preferential expression of tax/rex mRNA in the lung.
The similarity of proviral DNA load and quasispecies between PBMC and BALC strongly suggests the involvement of a tissue-specific condition conferring an advantage for viral expression in lung tissue. It was reported that HIV-1 RNA in mononuclear cells was more highly expressed in oesophageal mucosa than in lymph nodes, implicating local events promoting viral expression in ‘open’ organs such as the gastrointestinal tract [35]. The lung is also in contact with the exterior, and as such it may be subjected to local extracellular stimuli capable of activating cellular transcription factors that in turn regulate viral expression. Constitutive exposure to foreign antigens including other pathogenic microorganisms may cause direct activation of infected cells and stimulation of uninfected cells, including macrophages, to produce various chemokines and cytokines such as tumour necrosis factor and IL-2, which in turn activate infected T cells [1,8–10,36].
Viral expression is barely detectable in PBMC from HTLV-1-infected individuals in vivo, but is dramatically activated once cultured in vitro [37,38]. This suggests the presence of a factor inhibiting viral expression in serum of infected individuals [39]. Likely candidates for this role are serum antibodies to the virus. An alternative possibility is that a lower concentration of such an inhibitory factor in the lung would confer an advantage for viral expression. Elucidating the local events that promote HTLV-1 expression in the lung of HTLV-1-infected individuals will be critical to the understanding of its pathogenesis.
Acknowledgments
The authors thank Atsusi Yokoyama and Kenji Kurokawa for their excellent technical support, and Dr F. G. Issa (Word-Medex, Sydney, Australia) for editing of this manuscript.
REFERENCES
- 1.Watanabe T. HTLV-1-associated diseases. Int J Hematol. 1997;66:257–78. doi: 10.1016/s0925-5710(97)00077-7. [DOI] [PubMed] [Google Scholar]
- 2.Vermont JC, Buisson G, Magdeleine J, et al. T-lymphocyte alveolitis, tropical spastic paresis and Sjögren syndrome. Lancet. 1988;i:177. doi: 10.1016/s0140-6736(88)92744-4. [DOI] [PubMed] [Google Scholar]
- 3.Sugimoto M, Nakashima H, Watanabe S, et al. T-lymphocyte alveolitis in HTLV-1-associated myelopathy. Lancet. 1987;ii:1220. doi: 10.1016/s0140-6736(87)91362-6. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto M, Mita S, Tokunaga M, et al. Pulmonary involvement in human T lymphotropic virus type-1 uveitis: T-lymphocytosis and high proviral DNA load in bronchoalveolar lavage fluid. Eur Respir J. 1993;6:938–46. [PubMed] [Google Scholar]
- 5.Maruyama M, Tihara J, Sakashita I, et al. HTLV-1-associated bronchopneumonopathy: a new clinical entity? Am Rev Respir Dis. 1988;137:46. [Google Scholar]
- 6.Couderc LI, Caubarrere I, Venet A, et al. Bronchoalveolar lymphocytosis in patients with tropical spastic paraparesis associated with human T-cell lymphotropic virus type-1 (HTLV-1): clinical, immunologic, and cytologic studies. Ann Intern Med. 1988;109:625–8. doi: 10.7326/0003-4819-109-8-625. [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto M, Nakashima H, Matsumoto M, et al. Pulmonary involvement in patients with HTLV-1-associated myelopathy: increased soluble IL-2 receptors in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1989;139:1329–35. doi: 10.1164/ajrccm/139.6.1329. [DOI] [PubMed] [Google Scholar]
- 8.Noma T, Nakakubo H, Suguta M, et al. Expression of different combinations of interleukins by human T cell leukemia cell lines that are clonally related. J Exp Med. 1989;169:1853–8. doi: 10.1084/jem.169.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tendler CL, Greenberg SJ, Burton JD, et al. Cytokine induction in HTLV-1 associated myelopathy and adult T-cell leukemia: alternate molecular mechanisms underlying retroviral pathogenesis. J Cell Biochem. 1991;46:302–11. doi: 10.1002/jcb.240460405. [DOI] [PubMed] [Google Scholar]
- 10.Wucherpfenning KW, Hollsberg P, Richardson JH, et al. T-cell activation by autologous human T-cell leukemia virus type 1-infected T-cell clones. Proc Natl Acad Sci USA. 1992;89:2110–4. doi: 10.1073/pnas.89.6.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto M, Sugimoto M, Nakashima H, et al. Spontaneous T cell proliferation and release of soluble interleukin-2 receptors in patients with HTLV-1-associated myelopathy. Am J Trop Med Hyg. 1990;42:365–73. doi: 10.4269/ajtmh.1990.42.365. [DOI] [PubMed] [Google Scholar]
- 12.Mukae H, Kohno S, Morikawa N, et al. Increase in T-cells bearing CD25 in bronchoalveolar lavage fluid from HAM/TSP patients and HTLV-1 carriers. Microbiol Immunol. 1994;38:55–62. doi: 10.1111/j.1348-0421.1994.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 13.Higashiyama Y, Katamine S, Kohno S, et al. Expression of human T lymphotropic virus type 1 (HTLV-1) tax/rex gene in fresh bronchoalveolar lavage cells of HTLV-1-infected individuals. Clin Exp Immunol. 1994;96:193–201. doi: 10.1111/j.1365-2249.1994.tb06541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson S, Shida H, Macfarlin DE, et al. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-1 pX in patients with HTLV-1-associated neurological disease. Nature. 1990;348:245–51. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 15.Kannagi M, Harada S, Maruyama Y, et al. Predominant recognition of human T cell leukemia virus type 1 (HTLV-1) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-1-infected cells. Int Immunol. 1991;3:761–7. doi: 10.1093/intimm/3.8.761. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi I, Kubonishi I, Yoshimoto S, et al. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–1. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita T, Shimoyama M, Tobinai K, et al. Detection of mRNA for the tax/rex gene of human T-cell leukemia virus type-1 in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:5620–4. doi: 10.1073/pnas.86.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawase K, Katamine S, Moriuchi R, et al. Maternal transmission of HTLV-1 other than through breast milk: discrepancy between the polymerase chain reaction positivity of cord blood samples for HTLV-1 and the subsequent seropositivity of individuals. Jpn J Cancer Res. 1992;83:968–77. doi: 10.1111/j.1349-7006.1992.tb02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka M, Herr W. Differential transcriptional activation by Oct-1, and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990;60:375–86. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- 20.Seiki M, Hattori S, Hirayama Y, et al. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–22. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JD, Higgins DG, Gibson TJ, Clustal W. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 23.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 24.Page Rdm. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–8. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 25.Mita S, Sugimoto M, Nakamura M, et al. Increased human T lymphotropic virus type-1 (HTLV-1) proviral DNA in peripheral blood mononuclear cells and bronchoalveolar lavage cells from Japanese patients with HTLV-1-associated myelopathy. Am J Trop Med Hyg. 1993;48:170–7. doi: 10.4269/ajtmh.1993.48.170. [DOI] [PubMed] [Google Scholar]
- 26.Tajima K, Tominaga S, Suchi S, et al. Epidemiological analysis of the distribution of antibodies to adult T-cell leukemia-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Jpn J Cancer Res. 1982;73:893–901. [PubMed] [Google Scholar]
- 27.Hino S, Yamaguchi K, Katamine S, et al. Mother to child transmission of human T-cell leukemia virus type-1. Jpn J Cancer Res. 1985;76:474–80. [PubMed] [Google Scholar]
- 28.Komurian F, Pelloquin F, de The G. In vitro genomic variability of human T-cell leukemia virus type I depends more upon geography than pathogenesis. J Virol. 1991;65:3770–8. doi: 10.1128/jvi.65.7.3770-3778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niewiesk S, Bangham Cr. Evolution in a chronic RNA virus infection: selection on HTLV-1 tax protein differs between healthy carriers and patients with tropical spastic paraparesis. J Mol Evol. 1996;42:452–8. doi: 10.1007/BF02498639. [DOI] [PubMed] [Google Scholar]
- 30.Niewiesk S, Daenke S, Parker CE, et al. The transactivator gene of human T-cell leukemia virus type-1 is more variable within and between healthy carriers than patients with tropical spastic paraparesis. J Virol. 1994;68:6778–81. doi: 10.1128/jvi.68.10.6778-6781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito M, Furukawa Y, Kubota R, et al. Frequent mutation in pX region of HTLV-1 is observed in HAM/TSP patients, but is not specifically associated with the central nervous system lesions. J Neurovirol. 1995;1:286–94. doi: 10.3109/13550289509114025. [DOI] [PubMed] [Google Scholar]
- 32.Semmes OJ, Jeang Kt. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–92. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MR, Greene Wc. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Devel. 1990;4:1875–85. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 34.Inoue J, Yoshida M, Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type-1 genes. Proc Natl Aca Sci USA. 1987;84:3653–7. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith PD, Fox CH, Masur H, et al. Quantitative analysis of mononuclear cells expressing Human Immunodeficiency Virus Type-1 RNA in esophageal mucosa. J Exp Med. 1994;180:1541–6. doi: 10.1084/jem.180.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umehara F, Izumo S, Takeya M, et al. Expression of adhesion molecules and monocyte chemoattractant protein-1 (MCP-1) in the spinal cord lesions in HTLV-1-associated myelopathy. Acta Neuropathol. 1996;91:343–50. doi: 10.1007/s004010050435. [DOI] [PubMed] [Google Scholar]
- 37.Itoyama Y, Minato S, Kira J, et al. Spontaneous proliferation of peripheral blood lymphocytes increased in patients with HTLV-1-associated myelopathy. Neurology. 1988;38:1302–7. doi: 10.1212/wnl.38.8.1302. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson S, Gupta A, Mattoson D, et al. Immunological studies in tropical spastic paraparesis. Ann Neurol. 1990;27:149–56. doi: 10.1002/ana.410270209. [DOI] [PubMed] [Google Scholar]
- 39.Minato S, Itoyama Y, Goto I, Yamamoto N. Expression of HTLV-1 antigen in cultured peripheral blood mononuclear cells from patients with HTLV-1 associated myelopathy. J Neurol Sci. 1988;87:233–44. doi: 10.1016/0022-510x(88)90248-1. [DOI] [PubMed] [Google Scholar]


