Abstract
Pathogenic mechanisms of the demyelinating encephalopathy featuring the nervous phase of human African trypanosomiasis (HAT) are largely unknown. They might include autoimmune disorders. A variety of autoantibodies is detected during the disease and we have previously evidenced anti-galactocerebroside (GalC) antibodies in the serum and cerebrospinal fluid (CSF) from patients in the nervous stage (stage II) of HAT. We now show that anti-GalC antibodies recognize an antigen located on the parasite membrane and common to different strains of trypanosomes. By using affinity chromatography with a rabbit anti-GalC antiserum, a 52-kD proteolipid was isolated from the membrane of Trypanosoma brucei (T. b.) brucei AnTat 1.9, AnTat 1.1E, and T. b. rhodesiense Etat 1.2/R and Etat 1.2/S. Antibodies directed against this antigen were found in the CSF from patients with nervous stage HAT. These CSF also contained anti-GalC antibodies and adsorption with the proteolipid decreased anti-GalC reactivity. Immunization of mice with this antigen induced the production of antibodies which cross-reacted with GalC but no protection from experimental infection with T. b. brucei. These data support the hypothesis that anti-GalC antibodies detected in the CSF from HAT patients might be induced by molecular mimicry with a parasite antigen.
Keywords: galactocerebrosides, autoantibodies, molecular mimicry, Trypanosoma brucei
INTRODUCTION
Human African trypanosomiasis (HAT) (sleeping sickness) is caused by Trypanosoma brucei (T. b.) gambiense or T. b. rhodesiense. Its first stage lacks any involvement of the central nervous system (CNS), whereas stage II is characterized by the presence of parasites in the CNS and neurological disorders. Demyelination occurs during the terminal phases of the disease. The mechanisms leading to these disorders are still largely unknown [1–3]. CNS lesions observed both in human and experimental disease are featured by lymphocyte infiltrates in the meninges and by astrocytic and microglial hyperplasia in the brain [4,5]. B cell activation and production of autoantibodies, such as antibodies directed against nucleic acids, erythrocytes [6], cardiolipids [7] and intermediate filaments [8], are commonly observed. Autoantibodies specific for CNS components described during stage II of HAT including antibodies to myelin basic protein [9] or neurofilaments [10]. We also evidenced antibodies directed against galactocerebrosides (GalC), the major glycosphingolipids of the CNS, in an experimental model of HAT [11] and in the serum and cerebrospinal fluid (CSF) obtained from HAT patients [12,13]. CSF anti-GalC antibodies were characteristic of stage II of HAT. Such autoantibody production might result from the release of self-epitopes from damaged tissues. It might also reflect non-specific B cell stimulation secondary to hyperstimulation by parasitic antigens and inflammation. However, we suggested that certain autoantibodies produced during HAT might react with epitopes shared by trypanosome antigens and cellular structures [10]. The characterization of such cross-reacting antibodies might allow the identification of non-variant antigens of trypanosomes. In addition, it might provide data useful for a better understanding of the pathogenic mechanisms of the disease. We therefore searched for cross-reactivity of anti-GalC antibodies with a parasite antigen. Indeed, these antibodies recognized a conserved proteolipidic component of the parasite membrane, which suggests that anti-GalC antibodies might be induced by a molecular mimicry mechanism.
MATERIALS AND METHODS
Trypanosomes
Thin-blood films or centrifugation pellets of T. b. brucei (AnTat 1.1E, AnTat 1.2), T. b. gambiense (LiTat 1.1 to LiTat 1.10), T. b. rhodesiense (AnTat 25.1, ETat 1.2/R, ETat 1.2/S) and T. evansi (RoTat 1.2) were kindly provided by the Central Serum Bank for Sleeping Sickness (TDR/WHO project, Institute of Tropical Medicine, Antwerpen, Belgium).
Bloodstream form trypanosomes of the strain T. b. brucei AnTat 1.9 were cultured in minimal essential medium (MEM) with Earle’s salts supplemented with 2 mm glutamine, 25 mm HEPES, 0·2% glucose, 2 mm sodium pyruvate, 150 U/l penicillin (Gibco, Paisley, UK), 0·1 mm hypoxanthine, 0·01 mm thymidine, 0·5 mm 2-mercaptoethanol (2-ME; Sigma, St Louis, MO), 20% horse serum (Boehringer, Mannheim, Germany), 50 mm bathocuproine sulphate, and 1·5 mm l-cysteine. Medium was changed every 2 days.
Parasite extracts
Parasites were centrifuged at 500 g for 15 min, pellets were collected and either frozen for lipid extraction or lysed for Western blotting. Total extracts were prepared by lysis of cultured T. b. brucei AnTat 1.9 by freezing and thawing before resuspension in Laemmli buffer (62 mm Tris–HCl buffer pH 6·8, containing 2% SDS, 5% 2-ME, 10% glycerol, 0·01% bromophenol blue). Lipids were extracted from trypanosome pellets lysed by three freeze/thaw cycles by gentle agitation for 3 h at 4°C in chloroform–methanol (2:1, v/v). After filtration, solvents were evaporated under a nitrogen stream at 60°C and the total lipid extract was resuspended in methanol for thin-layer chromatography (TLC) or in Tris −1% SDS for Western blotting. The extraction procedure for antigen purification was slightly different (see below).
Indirect immunofluorescence
Various strains of trypanosomes (T. b. brucei: AnTat 1.9, AnTat 1.2, AnTat 1.1E; T. b. gambiense: LiTat 1.1 to LiTat 1.10; T. evansi: RoTat 1.2; T. b. rhodesiense: AnTat 25.1) were fixed using 4% paraformaldehyde in PBS, saturated with 10% normal goat serum and incubated for 1 h with rabbit anti-GalC antiserum (1:100; Sigma). Biotinylated goat anti-rabbit immunoglobulin antibodies (1:200, 30 min) and streptavidin–FITC complex (1:100, 30 min) (Dako, Glostrup, Denmark) were used as revelators. Non-specific binding of conjugated antibody was controlled by omitting primary antibodies. The other negative control was a normal rabbit serum (Sigma) studied under the same conditions.
Purification of trypanosome antigens by affinity chromatography
The same procedure was used for T. b. brucei (AnTat 1.9 and AnTat 1.1E) and T. b. rhodesiense (ETat 1.2/R, ETat 1.2/S). Parasites were lysed for 10 min at 0°C in MME buffer (10 mm MOPS −1 mm MgSO4 −0·1 mm EGTA, pH 6·9) containing 1% Triton X-100, 100 mm PMSF, leupeptin 2 mg/ml, aprotinin 5 mg/ml. Lysates were centrifuged at 10 000 g for 5 min. Pellets corresponding to cytoskeleton and flagellum were removed and supernatants (containing membrane components) were collected for analysis, according to Deflorin et al. [14].
Protein A beads (Protein A HyperD F; Biosepra, Marlborough, MA) were washed three times in 0·1 m borate buffer pH 8·2, incubated for 2 h at room temperature with rabbit anti-GalC antiserum diluted in the same buffer and washed once with borate buffer and once with 0·2 m triethanolamine pH 8·2. One volume of complexed beads was incubated in 20 volumes of cross-linking buffer (0·2 m triethanolamine −15 mm dimethylpimelimidate). After 1 h at room temperature, reaction was stopped with one volume of 0·2 m ethanolamine pH 8·2. Anti-GalC antibody-conjugated beads were washed three times with borate buffer and incubated overnight at 4°C with trypanosome membrane extracts under gentle shaking. Beads were washed with PBS −0·1% NP40, then with PBS −0·1% NP40–1 m NaCl, and finally with PBS −0·05% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulphonate) −1 m NaCl (as adapted from the procedure described by Schneider et al. [15]). Bound anti-Gal C-reactive materials were eluted with 0·1 m glycin pH 2·5 and immediately neutralized with 1 m Tris pH 8·9. The eluted antigens were dialysed overnight against PBS −0·05% CHAPS at 4°C. Protein concentrations in the eluates were determined by Lowry’s method [16]. Purified antigens were used directly for analysis by Western blotting or submitted to lipid extraction in chloroform–methanol–water (10:10:3, v/v/v).
Enzymatic treatments
Purified antigens (equivalent to 106 trypanosomes) eluted from cultured T. b. brucei AnTat 1.9 were incubated overnight at 37°C with 2 μ g phosphatidylinositol-specific phospholipase C (PI–PLC) (Sigma) in 0·1 m Tris–HCl, pH 7·4, 0·1% sodium deoxycholate buffer. Reaction was stopped by addition of 0·1 m sodium acetate. Pronase (100 μ g/ml, type XI-S from Tritiruchium album; Sigma) digestion was performed in PBS containing 0·1 m cysteine and 0·1 m EDTA. Reaction was stopped by addition of 0·1 m sodium acetate. The antigen was also cleaved by incubation overnight at 37°C with α-galactosidase (50 U/ml; Sigma) in 0·1 m sodium acetate pH 5·5. The reaction was stopped by boiling for 5 min. Samples were directly used for Western blotting or extracted in chloroform–methanol for analysis by TLC.
Immunodetection after thin-layer chromatography
After evaporation, samples were resuspended in an appropriate volume of methanol and submitted to chromatography (equivalent to 5 × 106 trypanosomes by spot) on aluminium-backed TLC plates (silica gel 60; Merck, Darmstadt, Germany), in a solvent system made up of chloroform–methanol −0·25% CaCl2 (50:40:10; v/v/v). Ten micrograms of a standard mixture of type I and type II GalC from bovine brain (Sigma) were used as positive control. After migration, the dried plates were dipped in a solution of 0·05% polyisobutyl methacrylate (Aldrich, Milwaukee, WI) in n-hexane for 40 s and saturated for 30 min with 10% inactivated horse serum in PBS. The plates were incubated overnight (4°C) with rabbit anti-GalC antiserum (1:200) diluted in PBS containing 1·5% bovine serum albumin (BSA). After washing with PBS, the plates were further incubated with biotinylated anti-rabbit immunoglobulin antibodies (1:1000, Dako) for 90 min at room temperature, then with a streptavidin–peroxidase complex (1:1000, 1 h; Dako) and revealed with a 10% 4-chloro-1-naphtol-Tris solution [11].
Western blotting
Equivalents of 106 parasites per lane were loaded. As a control of the reactivity of rabbit anti-total variable surface glycoprotein (VSG) antibodies (directed against soluble and membranous VSG), 5 μ g of VSG from T. b. gambiense Litat 1.5 were used (antibodies and VSG were provided by the Central Serum Bank for Sleeping Sickness).
After separation by SDS −12% polyacrylamide gel electrophoresis, proteins were electrotransferred onto nitrocellulose sheets (HAHY; Millipore, Saint-Quentin-Yvelines, France). After saturation for 30 min in PBS −3% BSA, the blots were incubated overnight at room temperature with either rabbit anti-GalC antiserum (1:500), rabbit anti-total VSG antibody (1:500), anti-soluble VSG rabbit polyclonal antibody specific for the cross-reacting determinant (CRD) of T. b. rhodesiense IlTat 1.21 (1:200, kindly provided by L. Cardoso De Almeida, ULB, Rhode Saint Genese, Belgium) [17] or human CSF (1:100). After washing with cold PBS, peroxidase-conjugated goat anti-rabbit immunoglobulin (1:2000) or anti-human immunoglobulin (1:5000) antibodies (Amersham, Les Ulis, France) were incubated for 1 h and developed by diaminobenzidine solution or an enhanced chemiluminescence reaction kit (Covalab; Dako) with exposure to X-OMAT-AR Kodak film for 30 s.
ELISA
CSF from 42 stage I and 38 stage II HAT patients and 36 normal CSF obtained after informed consent were studied by ELISA. All CSF had been previously studied for anti-GalC antibodies. Only CSF from stage II patients contained anti-GalC antibodies [11,12]. CSF (diluted 1:50 in PBS −1·5% BSA) (and a rabbit anti-GalC antiserum diluted 1:200 as positive control) were studied by ELISA with GalC (1 μ g in 50 μ l of methanol/well) and trypanosome-purified antigens (from T. b. brucei AnTat 1.9 cultured form, or from T. b. rhodesiense ETat 1.2/R bloodstream form (1 μ g in 50 μ l of PBS/well)) coated on either Nunc Immunosorb or Maxisorp microtitration plates (Nunc, Roskilde, Denmark) adapted for lipidic or proteic antigens, respectively. The plates were saturated with PBS −3% BSA (200 μ l/well) for 30 min at 37°C. CSF or rabbit anti-GalC antiserum were incubated overnight at 4°C. After washing with PBS −0·05% Tween 20, biotinylated anti-rabbit (1:1000; Dako), anti-human (1:1000; Amersham), or anti-mouse immunoglobulin antibodies (1:1000; Dako) were added for 90 min at 37°C, and revealed using streptavidin–peroxidase complex (1:1000) developed with 0·4% o-phenylenediamine −0·1 m citrate. Optical densities (OD) were measured at 492 nm and corrected ODs were calculated by subtracting OD corresponding to non-specific binding to non-coated wells. Each sample was analysed in duplicate.
Immunoadsorptions
Purified trypanosome antigens were used to adsorb the antibodies in five CSF from stage II HAT patients. Antigens (10 μ g) were incubated for 48 h at room temperature with CSF diluted 1:100 in PBS −1·5% BSA (500 μ l). After centrifugation at 10 000 g for 30 min, antibody activities to GalC and to the purified trypanosome antigen were measured in supernatants by ELISA, as above. The specificity of the adsorption was controlled by adsorption of an irrelevant serum containing IgM anti-GM1 antibody [18]. The anti-GM1 activity was unaffected.
GalC-containing immunoadsorbent was prepared with cholesterol particles dispersed in water as previously described [12] and used in adsorption experiments with sera from five mice immunized with trypanosome antigen (see below). Particles lacking GalC were used as a negative control.
Immunization
Five Swiss mice (females, 6–8 weeks old) were injected three times by i.p. injections at 2-week intervals with the purified antigen from cultured T. b. b. AnTat 1.9 in the presence of Freund’s complete adjuvant (FCA) for the first injection, and Freund’s incomplete adjuvant (FIA) for the second and third injections. Four control mice were injected with adjuvant alone. Serum antibodies to the purified antigens and to GalC were measured by ELISA as above, using mouse sera diluted 1:100 revealed by goat biotinylated anti-mouse IgM (1:10 000) and anti-mouse IgG (1:20 000) antibodies (Sigma).
Immunized mice were injected intraperitoneally with 500 trypanosomes (T. b. b. AnTat 1.9 culture form). Parasitaemia was assessed by microscopic examination of blood drops from the tail.
Statistical analysis
Statistical analysis was performed using non-parametric Mann–Whitney U-test. P < 0·05 was considered significant.
RESULTS
Indirect immunofluorescence staining of African trypanosomes by anti-GalC antibodies
Rabbit anti-GalC antibodies strongly stained the membrane of the parasite body of all bloodstream forms of trypanosomes tested (Fig. 1), as well as of the cultured forms of T. b. brucei AnTat 1.9, which was hence further used to characterize the target antigen. In contrast, a normal rabbit serum did not stain any form of the parasite (Fig. 1).
Fig. 1.
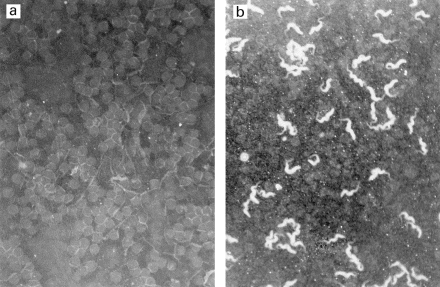
Staining of trypanosomes (T. b. brucei AnTat1–9) with rabbit anti-GalC antiserum (b) and normal rabbit serum (a) (indirect immunofluorescence, × 240).
Reactivity of anti-GalC antibodies with parasite extracts
The rabbit anti-GalC antiserum reacted with two components of T. b. brucei total lipid extracts by immunodetection after TLC. The first one migrated with the same referent front (Rf) as the standard GalC. The other reactivity was detected only after pronase treatment of trypanosome lipid extracts (data not shown), which suggests that this epitope was hidden on the TLC plate and unmasked by removal of the protein part of a proteolipidic structure. In contrast, Western blotting after electrophoresis under denaturating conditions of total lipid extracts showed a strong reactivity of rabbit anti-GalC antiserum with three proteins with apparent molecular weights of 38–52 kD (Fig. 2, lane 1).
Fig. 2.
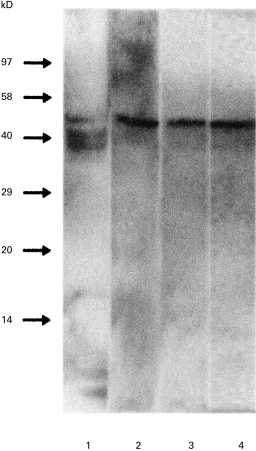
Characterization of the trypanosome-purified antigen by Western blotting. Total lipid extract of T. b. b. AnTat 1.9 (1); purified antigen from T. b. brucei AnTat 1.9 (2); lipid extracts from the purified antigen (3) and from the band recovered from the thin-layer chromatography (TLC) plate after migration of the antigen (4). Staining with rabbit anti-GalC antiserum.
Purification by affinity chromatography of the major antigen recognized by anti-GalC antibodies
Protein A bead-conjugated rabbit anti-GalC antibodies were used to purify the cross-reacting antigen(s) from the membranes of different parasite variants, T. b. brucei (AnTat 1–9, AnTat 1.1E) and T. b. rhodesiense (ETat 1.2/R, ETat 1.2/S). The eluted fraction accounted for about 0·4% of the total trypanosome proteins. By Western blotting and revelation with rabbit anti-GalC antibody, a 52-kD major protein was evidenced in all tested variants (Fig. 2, lane 2), and again the reactivity was detected by TLC only when the eluted fraction was treated with pronase. The eluted antigen solution was further lipid-extracted and studied by Western blotting. The same reactivity with a 52-kD component was found (Fig. 2, lane 3). Furthermore, the same reactivity was detected by Western blotting of a fraction recovered after TLC by scraping the silica in the Rf area determined above (Fig. 2, lane 4).
The antigen purified from trypanosome is a proteolipid
To confirm that the major antigen was a proteolipid, the reactivity of rabbit anti-GalC antiserum was studied after enzymatic treatment of parasite extracts. PI-PLC induced a shift of the migration by Western blotting (Fig. 3), which suggests that the antigen recognized by anti-GalC antibodies was a proteolipid containing a glycosyl‐phosphatidyl‐inositol (GPI) anchor to the membrane. As the major GPI-anchored proteins in trypanosomes are VSG, we studied the reactivity of anti-VSG antibodies with the antigen both native and treated by PI-PLC. Neither anti-total VSG antibodies nor anti-CRD antibodies reacted with any form of the antigen by Western blotting. In addition, treatment of the antigen by α-galactosidase abolished its reactivity with anti-GalC antibodies by Western blotting (data not shown), suggesting that α-Gal belongs to the epitope.
Fig. 3.
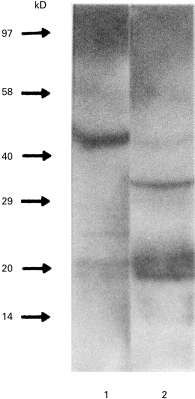
Western blotting of the purified antigen before (1) and after (2) phosphatidylinositol-specific phospholipase C (PI-PLC) treatment. Revelation using anti-GalC antibody.
Reactivity of patients’ CSF with T. b. brucei-purified antigen
The reactivity with the T. b. brucei AnTat 1.9 purified antigen of CSF from 42 patients in phase I, 38 patients in phase II and 36 normal subjects was also studied by ELISA. Rabbit anti-GalC antiserum, used as a positive control, showed a strong reactivity with coated Immunosorb plates (used for lipid coating because of its hydrophobic support), as well as with Maxisorp plates (used for protein coating because of hydrophilic interactions) (Fig. 4). CSF from stage II HAT patients contained significantly higher levels of antibody directed against the eluted antigen (mean corrected OD = 0·75 ± 0·28) than those from stage I patients (mean corrected OD = 0·41 ± 0·15; P < 0·0001) and normal subjects (mean corrected OD = 0·42 ± 0·12; P < 0·0001) (Fig. 4). As observed with rabbit anti-GalC antibody (see above), the antibody contained in CSF appeared to recognize a glycosylated epitope of the trypanosome antigen. Indeed, treatment with α-galactosidase strongly decreased the reactivity of 5 stage (II) by ELISA (75% reduction, P = 0·009).
Fig. 4.

Reactivity of human African trypanosomiasis (HAT) patients’ cerebrospinal fluids (CSF) with the purified antigen by ELISA: CSF (diluted 1:100) from phase I (PI), phase II (PII) patients and controls (N) (ELISA on Immunosorb plates). OD, Optical density.
Immunoadsorption
Five CSF from stage II HAT patients were adsorbed on the eluted antigen and the reactivity with GalC and trypanosome-purified antigen was controlled by ELISA before and after adsorption. Adsorption decreased IgM and IgG anti-GalC antibody reactivity by 27·8% (P = 0·028) and 42·5% (P = 0·009), respectively, as well as reactivity with the purified antigen (inhibition of IgM antibody = 21·5%, P = 0·047, and inhibition of IgG antibody = 46·8%, P = 0·009).
Immunization
Mice immunized with the eluted antigen developed high-level serum antibodies to the antigen purified from T. b. brucei AnTat 1.9, and from T. b. rhodesiense Etat 1.2/R, either on Maxisorp or Immunosorb plates. These antibodies predominantly belonged to the IgG class (P = 0·02 in comparison with control mice). Immunized mice also developed a weak antibody response to GalC (the difference with the controls was not significant) (Fig. 5). However, adsorption of these immune sera on GalC immunoadsorbent decreased their reactivity with the parasite antigen (23·5% inhibition for IgM antibody, P = 0·047; and 47% inhibition for IgG antibody, P = 0·009, respectively). Control adsorption experiments (with the adsorbent without GalC) were entirely negative. These data confirm that the antibody induced by immunization with the parasite antigen cross-reacted with GalC. These antibodies were not protective against experimental infection since immunized mice infected with T. b. brucei AnTat 1–9 died within 5–6 days, as did the control mice.
Fig. 5.
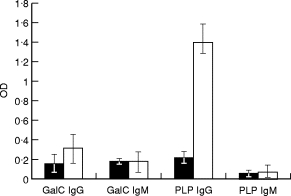
Serum antibody levels in mice immunized with the purified antigen. IgG and IgM antibodies to GalC (GalC IgG; GalC IgM) and to the proteolipidic purified antigen (PLP IgG; PLP IgM) in the sera from five immunized mice (□) (in comparison with four control mice (▪)). (ELISA on Immunosorb plates, means of corrected optical density (OD) ± s.d.).
DISCUSSION
Autoantibodies reacting with GalC are present in the CSF from stage II HAT patients [12,13]. The present study shows that such anti-GalC antibodies recognize a parasite membrane antigen, which is conserved among different variants and species of African trypanosomes. Affinity chromatography using rabbit anti-GalC antiserum allowed the purification of a 52-kD proteolipid. This epitope was dependent on the presence of carbohydrate residues (galactose). Anti-GalC antibody-containing CSF reacted with this antigen (and adsorption with the antigen decreased their reactivity with GalC also), in contrast to those from stage I patients (which did not contain detectable anti-GalC antibody either) and to normal CSF. Immunization of mice with the purified antigen elicited a strong IgG response with a weak cross-reaction with GalC. Such an IgG response directed against carbohydrate epitopes of glycosphingolipids is known to occur under certain experimental conditions [19] and in autoimmune neuropathies induced by molecular mimicry with a carbohydrate epitope of Campylobacter jejuni[20]. Altogether, the present data support the hypothesis that anti-GalC antibodies detected in HAT patients may result from a mechanism of molecular mimicry with the trypanosome antigen.
GalC is a major component of the myelin sheath. It is also expressed on the membrane of the myelinating cell of the CNS, the oligodendrocyte. GalC has been implicated in different signalling pathways, which affect the morphology and metabolism of oligodendrocytes. In association with myelin basic protein (MBP), it influences microtubule stability [21]. Binding of antibody to GalC induces Ca2 + influx and subsequent microtubule depolymerization [22,23]. It also appears that disrupted GalC synthesis alters the function of the myelin membrane [24]. GalC antigenic moiety involves carbohydrates [25]. Antibodies reactive with the epitope Gal α1–3 Gal have been described in another disease due to a trypanosome, namely Chagas’ disease. The latter antibodies cross-react with parasite antigens [26,27] and they may play a role in the protection against T. cruzi infection by a complement-mediated lysis of parasites [28,29]. Trypanosoma cruzi shares epitopes with astrocytes and neurones [30] and an autoimmune mechanism might participate in the involvement of the peripheral nervous system in Chagas’ disease. High titres of antigluco- and galactocerebroside antibodies have been reported in various conditions, including human visceral and cutaneous leishmaniasis, infection with T. rangeli and T. cruzi[31] and Mycoplasma pneumoniae encephalitis [32]. In murine experimental chronic infections by T. b. brucei, titres of antibodies to CNS antigens (MBP, GalC and gangliosides) correlate with CNS lesions [9]. Several experiments support the hypothesis that anti-GalC antibodies may be involved in certain demyelinating processes. The intraneural injection of anti-GalC antibody induces demyelination [33,34]. In experimental allergic encephalitis, demyelination occurs only after immunization with GalC in addition to MBP [35]. In vitro, anti-GalC antibodies are able to induce an antibody-dependent cellular cytotoxicity against oligodendrocytes [35–37]. However, it was also suggested that anti-GalC antibodies (and anti-MBP antibodies) could be merely secondary to neural tissue damage during multiple sclerosis and inflammatory polyneuropathies [38].
The lack of reactivity of anti-VSG antibodies with the purified antigen and its expression by the various trypanosome variants tested suggest that it might be related to invariant surface glycoproteins (ISG). Several ISG are known, including receptors and transporters, and they appear to be poorly accessible to antibodies because of their location in the flagellar pocket [39]. In contrast, in the present study the trypanosome antigen appeared to be expressed on the whole parasite membrane (as shown by immunofluorescent staining), with a probable GPI anchor.
Altogether, the present findings suggest that anti-GalC autoantibodies are induced by molecular mimicry with an invariant trypanosome antigen. The pathogenicity of these antibodies in the CNS involvement in HAT is an appealing hypothesis, which remains to be demonstrated.
Acknowledgments
This study was supported by grants from Conseil Régional du Limousin and the Ministère Français des Affaires Etrangères.
References
- 1.Greenwood BM, Whittle HC. The pathogenesis of sleeping sickness. Trans Roy Soc Trop Med Hyg. 1980;74:716–25. doi: 10.1016/0035-9203(80)90184-4. [DOI] [PubMed] [Google Scholar]
- 2.Adams JH, Haller L, Boa FY, Doua F, Dago A, Konian K. Human African trypanosomiasis (T. b. gambiense). A study of 16 fatal cases of sleeping sickness with some observations on acute arsenical encephalopathy. Neuropath Appl Neurobiol. 1986;12:81–94. doi: 10.1111/j.1365-2990.1986.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 3.Pentreath VW. Trypanosomiasis and the nervous system. Pathology and immunology Trans Roy Soc Trop Med Hyg. 1995;89:9–15. doi: 10.1016/0035-9203(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 4.Schultzberg M, Ambatsis M, Samuelsson EB, Kristensson K, van Meirvenne N. Spread of Trypanosoma brucei to the nervous system: early attack on the circumventricular organs and sensory ganglia. J Neurol Sci Res. 1988;21:56–61. doi: 10.1002/jnr.490210109. [DOI] [PubMed] [Google Scholar]
- 5.Keita M, Bouteille B, Enanga B, Vallat JM, Dumas M. Trypanosoma brucei brucei: a long term model of human African trypanosomiasis, meningo-encephalitis, astrocytosis, and neurological disorders. Exp Parasitol. 1997;85:183–92. doi: 10.1006/expr.1996.4136. [DOI] [PubMed] [Google Scholar]
- 6.Kobayakawa T, Louis J, Izui S, Lambert PH. Autoimmune response to DNA, red cells, and thymocytes antigens in association with polyclonal antibody synthesis during experimental African trypanosomiasis. J Immunol. 1979;122:296–301. [PubMed] [Google Scholar]
- 7.McKenzie AR, Boreham PFL. Autoimmunity in trypanosome infection. I. Tissue antibodies in Trypanosoma brucei infections of the rabbit. Immunology. 1974;26:1225–38. [PMC free article] [PubMed] [Google Scholar]
- 8.Anthoons JAMS, Van Marck EAE, Gigase PIJ. Autoantibodies to intermediate filaments in experimental infections with Trypanosoma brucei gambiense. Z Parasitenk. 1986;72:443–52. doi: 10.1007/BF00927888. [DOI] [PubMed] [Google Scholar]
- 9.Hunter CA, Jennings FW, Tierney JF, Murray M, Kennedy PGE. Correlation of autoantibody titres with central nervous system pathology in experimental African trypanosomiasis. J Neuroimmunol. 1992;41:143–8. doi: 10.1016/0165-5728(92)90064-r. [DOI] [PubMed] [Google Scholar]
- 10.Ayed Z, Brindel I, Bouteille B, Van Meirvenne N, Doua F, Houinato D, Dumas M, Jauberteau MO. Detection and characterization of autoantibodies directed against neurofilament proteins in human African trypanosomiasis. Am J Trop Med Hyg. 1997;57:1–6. [PubMed] [Google Scholar]
- 11.Jauberteau MO, Younes-Chennoufi AB, Amevigbe M, Bouteille B, Dumas M, Breton JC, Baumann N. Galactocerebrosides are antigens for immunoglobulins in sera of an experimental model of trypanosomiasis in sheep. J Neurol Sci. 1991;101:82–86. doi: 10.1016/0022-510x(91)90020-8. [DOI] [PubMed] [Google Scholar]
- 12.Amevigbe MDD, Jauberteau-Marchan MO, Bouteille B, Doua F, Breton JC, Nicolas JA, Dumas M. Human African Trypanosomiasis: presence of antibodies to galactocerebrosides. Am J Trop Med Hyg. 1992;47:652–62. doi: 10.4269/ajtmh.1992.47.652. [DOI] [PubMed] [Google Scholar]
- 13.Jauberteau MO, Bisser S, Ayed Z, et al. Détection d’autoanticorps anti-galactocérébrosides au cours de la trypanosomose humaine africaine. Bull Soc Path Ex. 1994;87:333–6. [PubMed] [Google Scholar]
- 14.Deflorin J, Rudolf M, Seebeck T. The major components of the paraflagellar rod of Trypanosoma brucei are two similar, but distinct proteins which are encoded by two different gene loci. J Biol Chem. 1994;269:28745–51. [PubMed] [Google Scholar]
- 15.Schneider C, Newman RA, Sutherland DR, Asser U, Greaves MF. A one step purification of membrane protein using a high efficiency immunomatrix. J Biol Chem. 1982;257:10766–9. [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 17.Cardoso de Almeida ML, Turner MJ. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983;302:349–52. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- 18.Jauberteau MO, Gualde N, Preud’Homme JL, Rigaud M, Gil R, Vallat JM, Baumann N. Human monoclonal IgM with autoantibody activity against two gangliosides (GM1 and GD1b) in a patient with motor neuron syndrome. Clin Exp Immunol. 1990;80:186–91. doi: 10.1111/j.1365-2249.1990.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamins JA, Callahan RE, Montgomery IN, Studzinski DM, Dyer CA. Production and characterization of high titer antibodies to galactocerebroside. J Neuroimmunol. 1987;14:325–38. doi: 10.1016/0165-5728(87)90019-1. [DOI] [PubMed] [Google Scholar]
- 20.Yuki N. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain–Barre syndrome and Miller Fisher syndrome. J Infect Dis. 1997;176(Suppl. 2):S150–3. doi: 10.1086/513800. [DOI] [PubMed] [Google Scholar]
- 21.Dyer CA, Philibotte TM, Wolf MK, Billings-Gagliardi S. Myelin basic protein mediates extracellular signals that regulate microtubule stability in oligodendrocyte membrane sheets. J Neurosci Res. 1994;39:97–107. doi: 10.1002/jnr.490390112. [DOI] [PubMed] [Google Scholar]
- 22.Dyer CA, Benjamins JA. Glycolipids and transmembrane signaling: antibodies to galactocerebroside cause an influx of calcium in oligodendrocytes. J Cell Biol. 1990;111:625–33. doi: 10.1083/jcb.111.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyer CA, Benjamins JA. Galactocerebroside and sulfatide independently mediate Ca2 + responses in oligodendrocytes. J Neurosci Res. 1991;30:699–711. doi: 10.1002/jnr.490300414. [DOI] [PubMed] [Google Scholar]
- 24.Bosio A, Binczek E, Stoffel W. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc Natl Acad Sci USA. 1996;93:13280–5. doi: 10.1073/pnas.93.23.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhat S. Galactose to ceramide linkage is essential for the binding of a polyclonal antibody to galactosyl ceramide. J Neuroimmunol. 1992;41:105–10. doi: 10.1016/0165-5728(92)90201-u. [DOI] [PubMed] [Google Scholar]
- 26.Avila JL, Rojas M, Galili U. Immunogenic Galα1–3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J Immunol. 1989;142:2828–34. [PubMed] [Google Scholar]
- 27.Souto-Padron T, Almeida IC, de Souza W, Travassos LR. Distribution of a-galactosyl-containing epitopes on Trypanosoma cruzi trypomastigote and amastigote forms from infected Vero cells detected by Chagasic antibodies. J Euk Microbiol. 1994;41:47–54. doi: 10.1111/j.1550-7408.1994.tb05933.x. [DOI] [PubMed] [Google Scholar]
- 28.Gazzinelli RT, Pereira ME, Romanha A, Gazzinelli G, Brener Z. Direct lysis of Trypanosoma cruzi: a novel effector mechanism of protection mediated by human anti-gal antibodies. Parasite Immunol. 1991;13:345–56. doi: 10.1111/j.1365-3024.1991.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 29.Almeida IC, Milani SR, Gorin PA, Travassos LR. Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-α-galactosyl antibodies. J Immunol. 1991;146:2394–400. [PubMed] [Google Scholar]
- 30.Petry K, Voisin P, Baltz T, Labouesse J. Epitopes common to trypanosomes (T. cruzi, T. dionisii and T. vespetilionis [Schizotripanum]): astrocytes and neurons. J Neuroimmunol. 1987;16:237–52. doi: 10.1016/0165-5728(87)90078-6. [DOI] [PubMed] [Google Scholar]
- 31.Avila JL, Rojas M. Elevated cerebroside antibody levels in human visceral and cutaneous leishmaniasis, Trypanosoma rangeli infection, and chronic Chagas’ disease. Am J Trop Med Hyg. 1990;43:52–60. doi: 10.4269/ajtmh.1990.43.52. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura M, Saida T, Kuroki S, Kawabata T, Obayashi H, Saida K, Uchiyama T. Post-infectious encephalitis with anti-galactocerebroside antibody subsequent to Mycoplasma pneumoniae infection. J Neurol Sci. 1996;140:91–95. doi: 10.1016/0022-510x(96)00106-2. [DOI] [PubMed] [Google Scholar]
- 33.Saida T, Saida K, Silberberg DH, Brown MJ. Transfer of demyelination by intraneural injection of experimental allergic neuritis serum. Nature. 1978;272:639–41. doi: 10.1038/272639a0. [DOI] [PubMed] [Google Scholar]
- 34.Saida K, Saida T, Brown MJ, Silberberg DH. In vivo demyelination induced by intraneural injection of anti-galactocerebroside serum. Am J Pathol. 1979;95:99–116. [PMC free article] [PubMed] [Google Scholar]
- 35.Krzalic LJ, Nedeljkovic DR, Cvetkovic DH, Skender MK. Serum lipids and demyelination in experimental allergic encephalomyelitis induced by the increased encephalitogenicity of myelin basic protein given with galactocerebroside. J Neuroimmunol. 1989;22:193–9. doi: 10.1016/0165-5728(89)90017-9. [DOI] [PubMed] [Google Scholar]
- 36.Griot-Wenk M, Griot C, Pfister H, Vandevelde M. Antibody-dependent cellular cytotoxicity in anti-myelin antibody-induced oligodendrocyte damage in vitro. J Neuroimmunol. 1991;33:145–55. doi: 10.1016/0165-5728(91)90058-f. [DOI] [PubMed] [Google Scholar]
- 37.Menon KK, Piddlesden SJ, Bernard CCA. Demyelinating antibodies to myelin oligodendrocyte glycoprotein and galactocerebroside induce degradation of myelin basic protein in isolated human myelin. J Neurochem. 1997;69:214–22. doi: 10.1046/j.1471-4159.1997.69010214.x. [DOI] [PubMed] [Google Scholar]
- 38.Wirguin I, Brenner T, Steinitz M, Abramsky O. In vitro synthesis of antibodies to myelin antigens by Epstein–Barr virus transformed B lymphocytes from patients with neurologic disorders. J Neurol Sci. 1991;104:92–96. doi: 10.1016/0022-510x(91)90221-r. [DOI] [PubMed] [Google Scholar]
- 39.Borst P, Fairlamb AH. Surface receptors and transporters of Trypanosoma brucei. Ann Rev Microbiol. 1998;52:745–78. doi: 10.1146/annurev.micro.52.1.745. [DOI] [PubMed] [Google Scholar]


