Abstract
HIV type 1 expression was significantly up-regulated in chronically infected promonocytic cell line (U1) co-cultured with human umbilical vein endothelial cells (HUVEC). Virus replication, evaluated as supernatant p24 release, was higher when U1 were co-cultured with IL-1β-activated HUVEC than with unstimulated HUVEC. When non-adherent U1 were removed from co-cultures, the remaining U1 cells adherent to the endothelial monolayer still showed enhanced HIV replication in comparison with an equal number of U1 cultured alone. While addition of adhesion molecule blocking antibodies (anti-intercellular adhesion molecule-1 (ICAM-1), -vascular cell adhesion molecule-1 (VCAM-1), -CD18 and -very late antigen-4 (VLA-4)) strongly inhibited adherence of U1 cells to endothelial monolayers, such treatment resulted in only a partial reduction in p24 release. Furthermore, HIV replication in U1 cells was enhanced on culture in HUVEC-conditioned media. Such data suggest that soluble mediators secreted by endothelial monolayers may modulate HIV-1 expression. Indeed, addition of cytokine and chemokine antagonists to both U1/HUVEC co-cultures and to U1 cultured in HUVEC-conditioned media clearly down-regulated p24 release. Anti-IL-6, anti-tumour necrosis factor-alpha (TNF-α) and, particularly, anti-MCP-1 MoAbs reduced p24 release, while anti-IL-8 polyclonal antiserum and IL-1 receptor antagonist (IL-1Ra) had no significant effect. Thus, the interaction between HUVEC and infected monocytic cells up-regulates HIV-1 replication predominantly through production of endothelium-derived soluble factors including MCP-1, TNF-α and IL-6. This phenomenon may influence the passage of HIV-1 from latency to productive replication and enhance virus spreading during physiological and/or pathological contact of monocytes with endothelium.
Keywords: monocytes, endothelial cells, HIV, cytokines, chemokines
INTRODUCTION
Cells of monocytic lineage are important targets for HIV-1. As HIV-1 replication in these cells is non-cytopathic and persistent [1], they may act as a major viral reservoir. Previous studies have demonstrated perivascular accumulation of mononuclear cells, including monocytes and macrophages, in different organs (heart, skeletal muscle, lungs and central nervous system) of HIV-infected subjects [2–6]. These leucocytes play a role in the pathophysiology of clinical conditions associated with HIV-1 [7,8]. Furthermore, the ability of virus-infected monocytes to enter tissues and to either take up residence or infect other mononuclear cells may participate in seeding of virus to other tissue sites. Monocyte migration from blood to tissues and vice versa requires a close contact with endothelium and adhesion molecule engagement during adhesion and transmigration. Adhesion seems to be a phenomenon able per se to modulate genic expression in monocyte/macrophages by inducing cellular activation [9]. Pro-adhesive changes in monocyte surface phenotype have been described both in experimentally infected cells and in monocytes from HIV+ subjects [10–12]. In addition, Shattock et al. demonstrated that experimentally induced adhesion molecule cross-linking by specific antibodies increased HIV-1 expression in the chronically infected promonocytic cell line OM10.1 [13]. Previous studies have demonstrated that contact with endothelial cells enhanced HIV-1 replication in in vitro infected macrophages and chronically infected monocytic cell lines [14,15]. However, in these experimental models both endothelium adherent and non-adherent monocytes and activating stimuli were present throughout the culture period, thus the observed effect on HIV-1 expression could be related to several experimental variables. Indeed, cell activators like phorbol esters and lipopolysaccharide (LPS) used to activate endothelial cells also induce HIV replication in monocytic cells [16,17]. Furthermore, soluble factors potentially secreted by endothelial cells such as tumour necrosis factor-alpha (TNF-α), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF) and monocyte chemotactic protein-1 (MCP-1) [18–24] have potential to enhance HIV expression.
In this study we demonstrate a marked increase in virus replication when U1 were co-cultured with human umbilical vein endothelial cells (HUVEC) in the absence of other activating stimuli. In addition, HIV replication was enhanced in U1 cells incubated in HUVEC-conditioned media. Inhibition experiments with anti-adhesion molecule blocking antibodies or with cytokine/chemokine antagonists showed that HIV up-regulation was only partly related to adhesion molecule engagement. Such data demonstrate that secretion of soluble mediators of endothelial origin provided the predominant stimuli driving HIV replication in U1/HUVEC co-cultures. Subsequent studies using neutralizing antibodies demonstrated that proinflammatory cytokines IL-6 and TNF-α and, especially, the CC chemokine MCP-1, play a pivotal role in the modulation of HIV-1 in U1/endothelium co-cultures.
MATERIALS and METHODS
HUVEC isolation and culture
Human endothelial cells were isolated from umbilical cord vein, cultured and characterized as previously described [25,26]. Briefly, first passage HUVEC were detached with trypsin–EDTA (ICN Biomedicals Inc., Costa Mesa, CA) and seeded (about 2 × 104 cells/well) in flat-bottomed 96-well microplates (Falcon, Franklin Lakes, NJ). Cells at confluence were incubated in E199 medium (Flow Labs, Irvine, UK) supplemented with 20% fetal calf serum (FCS; Gibco, Grand Island, NY), endothelial cell growth factor (ECGF; homemade) 50 μg/ml, 1% l-glutamine (ICN Biomedicals) and antibiotics with or without IL-1β 10 U/ml (R&D Systems, Minneapolis, MN) for 24 h. Resting or activated endothelial monolayers were either co-cultured with U1 in different experimental models or incubated for 48 h to collect culture supernatants. These supernatants were tested for their cytokine/chemokine content and used as conditioned media in U1 experiments.
Monocytic cells
U1 is a chronically HIV-1-infected human promonocytic cell line obtained by limiting dilution cloning of U937 cells acutely infected with IIIB/LAI strain of HIV-1 [16]. Cells were maintained in log phase growth in RPMI 1640 (Gibco) plus 10% FCS, 1% l-glutamine and antibiotics, and were washed twice in PBS before using at suitable experimental concentrations.
Unstimulated or phorbol-12-myristate-13-acetate (PMA)-stimulated U1 cells were tested for expression of surface adhesion molecules in cytofluorometric assays, as described below.
HIV-1 expression by U1 cells was evaluated as p24 release in supernatants from cultures performed according to experimental design (see below).
Flow cytometric analysis
U1 promonocytic cells were cultured overnight in tissue culture flasks at a concentration of 105/ml in medium alone or in the presence of PMA 20 ng/ml (Sigma, St Louis, MO). Subsequently cells (2 × 105/sample) were centrifuged at 1700 rev/min and pellets were resuspended in 100 μl cold PBS containing 2% bovine serum albumin (BSA) and to which mouse MoAbs specific to human CD11a, CD11b, CD11c or CD18 adhesion molecules were added. Samples were incubated for 30 min at 4°C and washed three times before the addition of 2 μl of FITC-conjugated anti-mouse IgG antibody. After 30 min incubation at 4°C, cells were washed three times, fixed in PBS/paraformaldehyde 1% and analysed by flow cytometry in a FACScan system (Ortho, Raritan, NJ). Results were expressed as percentage of positive fluorescent cells.
U1/HUVEC in vitro culture models
U1/HUVEC co-cultures
U1 cells (105/well; 100 μl final volume) were seeded in flat-bottomed 96-well microplates on HUVEC monolayers. Both resting or IL-1β (10 U/ml for 24 h) activated HUVEC were used, after extensive washing. Co-cultures were performed in complete U1 medium (RPMI 1640 plus 10% FCS, 1% l-glutamine and antibiotics) for 48 h at 37°C and 5% CO2. The plates were then centrifuged at 1300 rev/min for 15 min and the supernatants collected for the measurement of p24 levels.
Co-cultures of adherent U1 and HUVEC
In order to investigate whether HIV-1 up-regulation was sustained only by adherent cells, 105 U1 were incubated on endothelial monolayers for 1 h. Subsequently non-adherent U1 were removed by extensive washing, and the remaining adherent cells incubated for a further 48 h, after which culture supernatants were collected as described above.
Parallel cultures were performed in order to evaluate the number of U1 effectively adherent to resting or IL-1β-activated HUVEC using 51Cr-release assay (see below). p24 release was also measured from a two-fold dilution series of U1 cells (starting from 105 cells/well). Having determined the number of U1 adherent to HUVEC monolayers, using the functional adhesion assay, data from the dilution series of U1 cultured alone were used to compare p24 release from adherent U1/HUVEC co-cultures with the same number of U1 cultured in the absence of endothelium.
U1 cultures in HUVEC-conditioned media
U1 were cultured in 96-well flat-bottomed plates for 48 h (105/well; 100 μl final volume) in the absence or presence of two-fold dilutions of supernatants derived from resting or IL-1β-activated HUVEC monolayers. After centrifugation, U1 culture supernatants were collected and tested for p24 content.
Functional adhesion assay
U1 cells (106/μl) were radiolabelled with 51Cr (sodium dichromate, 30 μCi/105 cells; Amersham Int. plc, Aylesbury, UK) for 1 h at 37°C and seeded (105/100 μl) in the presence of resting or activated HUVEC. After 1 h adhesion, plates were washed three times and the remaining cells lysed with 0·1% SDS/NaOH 0·025 m. Adhesion was measured in ct/min and expressed as percentage of adherent cells per total radiolabelled U1 originally seeded on HUVEC monolayers (100%), as described [27,28].
Adhesion molecule blocking experiments
U1/HUVEC (resting or IL-1β-activated) co-cultures were set up both with and without anti-adhesion molecule MoAbs. We used the following antibodies: anti-CD54/intercellular adhesion molecule-1 (ICAM-1) MoAb MCA532 (Serotec Ltd, Oxford, UK); anti-CD106/vascular cell adhesion molecule-1 (VCAM-1) MoAb 4B9, which is a blocking MoAb [29]; MoAb 60.3, which recognizes a functional epitope on the CD18 subunit (common β-chain) of the CD11/CD18 antigen complex [30]; MoAb HP2/1, which binds to a functional epitope on the CD49d (α-chain) of the leucocyte CD49d/CD29 (very late antigen-4 (VLA-4)) integrin [31] (gift of Dr John M. Harlan).
Preliminary observations confirmed that while all MoAbs used in this study induced partial inhibition of monocyte adherence to HUVEC, maximal inhibition of adhesion (13-fold reduction with activated HUVEC) was only obtained with a mixture of the MoAbs. Adhesion assays were performed as previously described [32].
All the MoAbs were used at 10 μg/ml final concentration. Cells, either U1 or resting and IL-1β-activated HUVEC, were preincubated with specific MoAbs or complete medium for 15 min; subsequently, U1 (105/well) were seeded with HUVEC in the presence of MoAb mixture or culture medium. Some U1/HUVEC co-cultures were incubated for 48 h, before collecting culture supernatants for p24 measurement. In parallel cultures non-adherent U1 were removed after 1 h adhesion and the residual adherent cells were co-cultured with endothelial monolayer for a further 48 h before harvesting. p24 levels were determined in culture supernatants and compared with either antigen content in the absence of MoAbs or secretion by equivalent U1 cultured alone.
Cytokine/chemokine neutralization experiments
Cytokine/chemokine antagonists were added to: U1/HUVEC (resting or IL-1β-activated) co-cultures in which non-adherent cells were removed, and U1 cells (105/well; 100 μl final volume) in HUVEC-conditioned medium. Neutralization experiments were carried out using MoAbs to IL-6 (0·5 μg/ml), TNF-α (1 μg/ml) (both by R&D Systems) and IL-1 receptor antagonist (IL-1Ra) 1 μg/ml (gift of Dr P. Bossù, Dompè S.p.A., L’Aquila, Italy). To affect potential activity of IL-8 and MCP-1 chemokine, a rabbit polyclonal antiserum anti-IL-8 (1:25 dilution) [33] and two mouse MoAbs to MCP-1: (5D3-F7, ascite, 1:250 dilution, in-house preparation) [34] and a blocking anti-MCP-1 MoAb (LS1; 5 μg/ml; Leukosite) were used. Cytokine/chemokine antagonist concentrations used in our experiments were derived from dose–response studies. p24 levels in 48 h culture supernatants in the presence of cytokine/chemokine antagonists were compared with antigen content in control cultures without antagonists.
ELISA
IL-1β, TNF-α, IL-6, IL-8 and MCP-1 content in culture supernatants was determined by commercial kits from R&D Systems; p24 release was determined by a Cellular Products Inc. (Buffalo, NY) kit. The assays were performed according to the manufacturer’s instructions.
Statistical analysis
Descriptive statistics of the differences between values were calculated as mean, s.d., minimum and maximum in the original measurement scale of the recorded variables (pg/ml) and reported in Results for results readability purposes.
For graphical presentation, common logarithm transformation of the data has been made.
Owing to the limited number of experimental units and the skewed distribution of the recorded variables, the non-parametric Friedman two-way analysis of variance for related samples was used. Then pairwise comparisons were performed by the Wilcoxon matched pairs signed rank test with the statistical significance threshold 0·05 accordingly corrected by the Bonferroni’s procedure [35].
RESULTS
U1 co-culture with HUVEC monolayers enhances HIV replication
We evaluated p24 levels secreted by U1 (105 cells/well) cultured in the absence or presence of resting or IL-1β-activated endothelial monolayers. In comparison with U1 cultured alone (105 cells/well), co-cultures with IL-1β-activated HUVEC strongly up-regulated p24 release (seven-fold increase, mean +s.d. 3112 + 1912 pg/ml; minimum and maximum 631 and 6425 pg/ml); an evident but lower up-regulation was also found in the presence of resting HUVEC (2·5-fold increase, 775 + 675 pg/ml; minimum and maximum 111 and 1802 pg/ml). In both conditions the increase in p24 levels was statistically significant (P < 0·025) (Fig. 1a). As shown in Fig. 1b, when non-adherent U1 were removed, an evident statistically significant (P < 0·01) virus up-regulation was still observed in the co-cultures compared with equal numbers of U1 cultured alone (about five-fold enhancement in resting conditions, 301 + 292 pg/ml; minimum and maximum 7 and 820 pg/ml; 14-fold increase after activation, 2574 + 1849 pg/ml; minimum and maximum 308 and 6220 pg/ml).
Fig. 1.
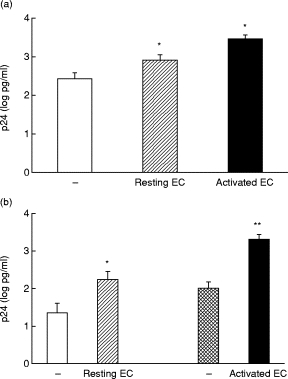
U1 co-culture with human umbilical vein endothelial cell (HUVEC) monolayers enhances HIV replication. (a) p24 levels in culture supernatants from U1 alone (105 cells/well) (□), U1 co-cultured with resting HUVEC (hatched) or activated HUVEC monolayers (▪). (b) p24 release from U1 cells adherent to resting HUVEC monolayers (hatched), activated HUVEC (▪) and equivalent numbers of U1 cultured alone (□ and cross-hatched). Data are expressed as p24 (log pg/ml), mean and s.e.m. of 10 experiments. *P < 0·025; **P < 0·01.
Control cultures of U1 stimulated by PMA showed a six-fold increase in p24 secretion while the proinflammatory cytokines IL-6, IL-1β and TNF-α induced a four-, 15- or 35-fold enhancement in viral antigen levels, respectively (data not shown). These data indicate that the up-regulation of HIV replication observed in our models of U1/HUVEC co-cultures is higher than, or at least similar to the increase induced by known stimulants.
Role of adhesion molecules in modulating p24 release from U1/HUVEC co-cultures
As cell–cell contact can influence HIV-1 expression in infected cells, we investigated the role of adhesion molecules in our in vitro co-culture models. U1 cells express high levels of CD11a and CD18 adhesion molecules both in resting and activation (PMA) conditions (> 95% positive cells). Basal expression of CD11b and CD11c was quite low (2·1% and 16·9% positive cells, respectively) and increased in response to PMA stimulus (25·7% and 68·3% positive cells, respectively). Adhesion molecule blocking experiments were carried out with a mixture of MoAbs specific for ICAM-1 (CD11a/CD18 ligand), VCAM-1 (VLA-4 ligand), CD18 and VLA-4. Functional adhesion, evaluated by the 51Cr assay, demonstrated that these antibodies significantly (P < 0·001) inhibited U1 adherence to resting or activated HUVEC by 42 ± 5% and 77 ± 9%, respectively (see Fig. 2a for adhesion to activated HUVEC).
Fig. 2.
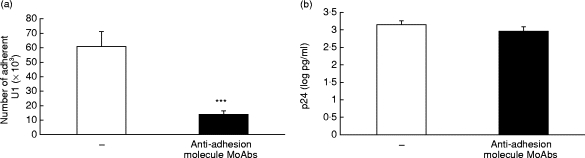
Effects of adhesion molecule MoAbs on adhesion and p24 release in U1/human umbilical vein endothelial cell (HUVEC) co-cultures. (a) Inhibition of U1 adhesion to activated HUVEC by adhesion molecule MoAbs. Number of adherent U1 in the absence (□) or in the presence (▪) of adhesion molecule MoAbs (specific form intercellular adhesion molecule-1, vascular cell adhesion molecule-1, very late antigen-4 and CD18). Data are expressed as number of adherent cells, mean and s.e.m. of seven experiments. ***P < 0·001. (b) Adhesion molecule MoAbs modulate p24 release from U1 cells co-cultured with activated HUVEC monolayers. p24 release from U1 co-cultured with activated HUVEC in the absence (□), or presence of adhesion molecule MoAbs (▪). Data are expressed as p24 (log pg/ml), mean and s.e.m. of seven experiments.
In contrast, addition of adhesion molecule MoAbs to U1 co-cultured with activated HUVEC resulted in only a partial reduction (29 ± 4%) in supernatant p24 release (Fig. 2b; decrease, 503 + 339 pg/ml; minimum and maximum 170 and 1119 pg/ml). A similar partial reduction in p24 release was seen on addition of MoAbs to U1 co-cultured with resting HUVEC, and when used in the co-culture model where non-adherent U1 cells were removed (data not shown). In all co-culture models, p24 release in the presence of MoAbs was higher than that seen with equivalent U1 cultured alone. Incubation of U1 alone with the adhesion molecule MoAb mixture had no effect on p24 release, ruling out the possibility of a non-specific effect of the MoAbs on HIV-1 replication (data not shown).
Role of soluble mediators in modulation of p24 release from U1/HUVEC co-cultures
To investigate the involvement of soluble factors in the modulation of HIV-1 expression observed in our co-culture model, we tested the effect of HUVEC-conditioned culture supernatants on p24 release by U1.
Culture of U1 cells in conditioned medium from resting or activated endothelial monolayers resulted in a two- and eight-fold (P < 0·005) enhancement in p24 release, respectively (Fig. 3; increase, 340 + 309 pg/ml; minimum and maximum 23 and 1105 pg/ml, in resting conditions; 2066 + 1990 pg/ml; minimum and maximum 305 and 7055 pg/ml, after activation). Of note, this increase is higher than that induced by PMA or exogenous IL-6 (four- and six-fold increase, respectively; data not shown).
Fig. 3.
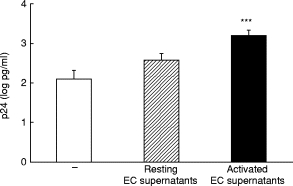
Effect of human umbilical vein endothelial cell (HUVEC)-conditioned media on U1 p24 production. p24 levels in culture supernatants from U1 cultured alone (105 cells/well) (□), or in two-fold dilutions of conditioned medium from resting (hatched) or activated HUVEC (▪). Data are expressed as p24 (log pg/ml), mean and s.e.m. of 10 experiments. ***P < 0·005.
To determine potential soluble factors modulating U1 p24 release, we assessed cytokine and chemokine content in the HUVEC-conditioned supernatants used for our experiments (Table 1). In line with previous reports, IL-1β-activated HUVEC did secrete much higher amounts of IL-6, TNF-α, IL-8 and MCP-1 in the supernatants than control resting cells. U1 cells themselves did not secrete detectable amounts of proinflammatory cytokines (IL-6, IL-1β and TNF-α), while basal MCP-1 levels in 48 h culture supernatants appear low (1600 ± 855 pg/ml, mean ± s.d. of six experiments), as previously reported [36].
Table 1.
Cytokine/chemokine content in supernatants from resting or IL-1β-activated human umbilical vein endothelial cell (HUVEC) monolayers
| Resting HUVEC | Activated HUVEC | |
|---|---|---|
| IL-6 (pg/ml) | 2500 ± 500 | 28 300 ± 3980 |
| IL-1β (pg/ml) | 0·10 ± 0·07 | 0·13 ± 0·1 |
| TNF-α (pg/ml) | 1·63 ± 0·6 | 3·50 ± 1·1 |
| IL-8 (pg/ml) | 5485 ± 977 | 14 460 ± 401 |
| MCP-1 (pg/ml) | 19 272 ± 4618 | 82 202 ± 13 092 |
Values represent mean ±s.d. of six experiments.
To evaluate the potential role of these different soluble mediators in modulating U1 p24 release, U1 cells were cultured in HUVEC-conditioned media in the presence of specific cytokine/chemokine antagonists. We observed a marked decrease in HIV-1 replication when anti-IL-6, -TNF-α and, of note, -MCP-1 (both 5D3-F7 and LS1) MoAbs were added to U1 cultured in conditioned media from activated endothelial cells (Fig. 4a). In particular MCP-1 blocking MoAbs significantly inhibited p24 from U1 cells to the level of basal expression (decrease, 1885 + 1410 pg/ml; minimum and maximum 309 and 3930 pg/ml, with 5D3-F7; and 1539 + 1226 pg/ml; minimum and maximum 266 and 3060 pg/ml, with LS1; P < 0·025 for both). In contrast, polyclonal anti-IL-8 antibodies and IL-1Ra did not affect p24 secretion in these cultures. Due to the lower levels of cytokine and chemokine release from resting HUVEC, comparable, but biologically less important, results were found with U1 cultured in conditioned media from such cells (data not shown). Addition of the same cytokine/chemokine antagonists to co-cultures of U1 cells adherent to activated HUVEC (non-adherent cells removed by washing) inhibited p24 release in a similar manner to that found with conditioned media (Fig. 4b; decrease, 3075 + 1414 pg/ml; minimum and maximum 1382 and 5262 pg/ml, with 5D3-F7; and 2504 + 1174 pg/ml; minimum and maximum 1102 and 4466 pg/ml, with LS1; P < 0·001 for both). The addition of cytokine antagonists did not modify the magnitude of inhibition induced by both anti-MCP-1 MoAbs and no additive effects were observed when cytokine antagonists were used in combination (data not shown). These observations together with the differences in antibody affinity and in the relative concentration of the mediators in our culture models make it difficult to draw any conclusion about the signal transduction pathway(s) involved in HIV expression modulation. However, such data strongly suggest a predominant role for soluble mediators in up-regulating HIV expression in U1/endothelial co-cultures.
Fig. 4.
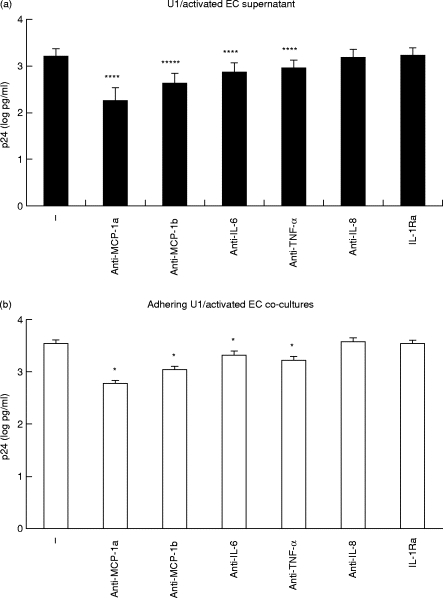
Modulation of U1 p24 release by cytokine/chemokine antagonists added to: (a) U1 cells cultured in conditioned media from activated human umbilical vein endothelial cells (HUVEC) and (b) co-cultures of U1 cells adherent to activated HUVEC monolayers. Anti-MCP-1a and anti-MCP-1b indicate the 5D3-F7 and the LS1 MoAbs, respectively. Results are expressed as p24 (log pg/ml), mean and s.e.m. of seven independent experiments. *P < 0·025; ****P < 0·001.
DISCUSSION
In this study we demonstrate that in vitro interaction between cells of the human HIV-1 chronically infected promonocytic line U1 and HUVEC resulted in a significant enhancement of HIV-1 replication, either in co-culture models or when U1 were incubated in HUVEC-conditioned media. This enhancing effect was higher than or similar to that induced by known stimulants and appeared to be due to both cell–cell contact and endothelial derived soluble mediators.
Fan et al. [14] previously reported an increase in p24 secretion by U1 co-cultured with resting or LPS-activated HUVEC monolayers, in a model in which a fixed number of monocytic cells were plated together with endothelium. As non-adherent U1 cells were not removed from these co-cultures, the relative contribution of adherent versus non-adherent U1 to the observed HIV-1 modulation was unclear. In addition, the presence of LPS throughout these experiments, a powerful activator of both U1 and endothelial cells, made it difficult to understand the mechanisms involved in the enhancement of HIV replication. In the studies described in this paper, both non-adherent U1 cells and exogenous activators were removed from U1/HUVEC co-cultures. Under such experimental conditions we observed heightened HIV-1 expression in adherent U1 cells. Adherence is a phenomenon able per se to activate cells by inducing mRNA expression for various proto-oncogenes and cytokines, such as TNF-α and IL-8 [37–39]. In cellular adhesion a variety of cellular and extracellular matrix proteins is involved [40–42]; among them, adhesion molecules of the integrin family are reported to be important, as they also mediate events that modify the state of cellular activation [43]. The regulatory signals mediated by adhesion molecule involvement may influence HIV production in infected cells. Previous studies report that adhesion molecule engagement, mediated by cross-linking antibodies, enhances HIV-1 replication in monocytic cells [13] and that CD11/CD18 integrins are involved in up-regulation of HIV-1 expression by monocytes interacting with HUVEC [14]. However, we observed that presence of a combination of MoAbs to CD18, VLA-4, ICAM-1 and VCAM-1, while strongly inhibiting U1 adhesion to HUVEC monolayers, resulted in only a partial down-regulation of p24 release. The incubation of U1 with anti-adhesion molecule MoAbs in the absence of endothelium did not significantly modify viral antigen release, indicating that the MoAbs do not interfere aspecifically with HIV-1 replication. These results suggest that integrin engagement during monocyte/HUVEC interaction plays only a minor role in modifying HIV release from U1 cells.
HIV-1 expression may also be influenced by endogenous cytokine production. Recently, Ho et al. [44] reported enhanced HIV-1 expression in U1 co-cultured with human lung fibroblasts, and this effect seemed to be mediated by IL-6 [45]. Furthermore, previous reports have also demonstrated that culture of a chronically infected promonocytic clone in astrocyte-conditioned medium enhanced HIV-1 replication [46]. Endothelial cells are known to produce proinflammatory cytokines including IL-6, TNF-α or GM-CSF [18–22], and CC chemokines (MCP-1) [24] with potential to up-regulate HIV replication. We studied cytokine/chemokine involvement in enhancement of HIV replication observed in our U1/HUVEC co-culture models using specific antagonists. We demonstrate that MoAbs to the cytokines IL-6, TNF-α and, more evidently, to the chemokine MCP-1 strongly inhibit enhanced HIV expression. To investigate the effect of soluble mediators independently of cell–cell adhesion, U1 were cultured in HUVEC-conditioned supernatants, containing high amounts of several proinflammatory cytokines and chemokines. In this culture model, HIV-1 expression also appeared to be clearly up-regulated, and this effect was significantly reduced by the addition of neutralizing antibodies to IL-6, TNF-α and MCP-1. Such findings indicate that soluble mediators of endothelial origin are the predominant factors enhancing HIV-1 expression in monocyte–endothelial co-cultures.
In our models of U1/HUVEC interaction the CC chemokine MCP-1 seems to play a pivotal role in inducing HIV-1 expression, demonstrated by the highly inhibitory effect of two different anti-MCP-1 MoAbs on p24 antigen. These findings are in line with those of Vicenzi et al., who demonstrated that MCP-1 was associated with heightened HIV-1 replication in vitro[24]. It is interesting to note that plasma MCP-1 levels have been reported to correlate with virus load in vivo[47]. Furthermore, selective increases in MCP-1 levels reported in the cerebrospinal fluid (CSF) of AIDS patients with cytomegalovirus encephalitis [48] or HIV encephalitis [49], correlate with local HIV RNA levels [50].
Results presented in this study suggest that the interaction between HIV-infected monocytes and endothelium may influence the passage of HIV-1 from latency to productive replication, through both cell–cell contact and, particularly, soluble factor secretion. Such interaction may be particularly significant because of the wide distribution of endothelium in the tissues and the continuous physiological contact of circulating monocytes with vessel walls. Data from this study could support potential therapeutic approaches aimed at controlling secretion of HIV-1-enhancing chemokines and/or at inhibiting their receptors. Indeed, experimental studies with MCP-1 antagonists have already shown their ability to treat inflammatory conditions such as arthritis in MLR-lprlpr mice [51].
Acknowledgments
We thank Professor F. Reina for his expert assistance in statistical evaluation of the data. This work was supported by grants N. 40 A0.66 and 40 A0.68 of the 1997 I National Programme on AIDS Research by Istituto Superiore di Sanità, Rome, Italy.
REFERENCES
- 1.McElrath MJ, Pruett JE, Cohn ZA. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoir on human immunodeficiency virus type I infection. Proc Natl Acad Sci USA. 1989;86:675–9. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batungwanayo J, Taelman H, Allen S, et al. Cardiac involvement in human immunodeficiency virus-infected patients. J Acquir Immune Defic Syndr. 1993;86:380–5. [PubMed] [Google Scholar]
- 3.Michel C, Dosquet P, Ronco P, et al. Tissue infiltration in a CD8 lymphocytosis syndrome associated with human immunodeficiency virus-I infection as the phenotypic appearance of an antigenically driven response. J Clin Invest. 1993;91:2216–25. doi: 10.1172/JCI116448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent state of disease. Nature. 1993;362:355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 5.Vazuex R, Brousse N, Jarre A, et al. AIDS subacute encephalitis: Identification of HIV-infected cells. Am J Pathol. 1987;126:403–10. [PMC free article] [PubMed] [Google Scholar]
- 6.Wiley CA, Schrier RD, Nelson JA, et al. Cellular localization of human immunodeficiency virus infection within the brains of acquired immunodeficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–93. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meltzer MS, Skillman DR, Gomatos PJ, et al. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–94. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- 8.Spencer DC, Price RW. Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol. 1992;46:655–93. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- 9.Sporn SA, Eierman DF, Johnson CE, et al. Monocyte adherence results in selective induction of novel genes sharing homology with mediators of inflammation and tissue repair. J Immunol. 1990;144:4434–41. [PubMed] [Google Scholar]
- 10.Dhawan S, Weeks BS, Soderland C, et al. HIV-1 infection alters monocyte interaction with human microvascular endothelial cells. J Immunol. 1995;154:422–32. [PubMed] [Google Scholar]
- 11.Trial J, Birdsall HH, Hallum JA, et al. Phenotypic and functional changes in peripheral blood monocytes during progression of immunodeficiency virus infection. J Clin Invest. 1995;95:1690–701. doi: 10.1172/JCI117845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birdsall HH, Trial J, Hallum JA, et al. Phenotypic and functional activation of monocytes in HIV-1 infection: interactions with neural cells. J Leukoc Biol. 1994;56:310–7. doi: 10.1002/jlb.56.3.310. [DOI] [PubMed] [Google Scholar]
- 13.Shattock RJ, Rizzardi GP, Hayes P, et al. Engagement of adhesion molecules (CD18, CD11a, CD45, CD44 and CD58) enhances HIV-1 replication in monocyte cells through a TNF modulated pathway. J Infect Dis. 1996;174:54–62. doi: 10.1093/infdis/174.1.54. [DOI] [PubMed] [Google Scholar]
- 14.Fan ST, Hsia K, Edgington TS. Upregulation of human immunodeficiency virus-1 in chronically infected monocytic cell line by both contact with endothelial cells and cytokines. Blood. 1994;84:1567–72. [PubMed] [Google Scholar]
- 15.Gilles PN, Lathey JL, Spector SA. Replication of macrophage-tropic and T-cell-tropic strains of human immunodeficiency virus type I is augmented by macrophage–endothelial cell contact. J Virol. 1995;69:2133–9. doi: 10.1128/jvi.69.4.2133-2139.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folks TM, Justement J, Kinter A, et al. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J Immunol. 1988;140:1117–22. [PubMed] [Google Scholar]
- 17.Pomerantz RJ, Feinberg MB, Trono D, et al. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human HIV-1 expression. J Exp Med. 1990;172:253–61. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor α and interleukin 1 stimulate the human immunodeficiency virus enhancer by the activation of the nuclear factor kb. Proc Natl Acad Sci USA. 1989;86:2336-40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin GE, Leung K, Folks TM, et al. Activation of HIV gene expression during monocyte differentiation by induction of NF-kB. Nature. 1989;3392:70–3. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- 20.Poli G, Bressler P, Kinter A, et al. Interleukin 6 induces HIV expression in infected monocyte cells alone and in synergy with TNFα by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–8. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folks TM, Justement J, Kinter A, et al. Cytokine-induced expression of HIV-1 in a chronically infected promonocytic cell line. Science. 1987;238:800–2. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 22.Koyanagi Y, O’Brien WA, Zhao JQ, et al. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1989;241:1673–5. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 23.Cocchi F, De Vico AL, Garzino-Demo A, et al. Identification of RANTES, MIP-1α and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 24.Vicenzi E, Biswas P, Mengozzi M, et al. Role of pro-inflammatory cytokines and β-chemokines in controlling HIV replication. J Leukoc Biol. 1997;62:34–40. doi: 10.1002/jlb.62.1.34. [DOI] [PubMed] [Google Scholar]
- 25.Ferraro G, Meroni PL, Del Papa N, et al. In vitro modulation of human endothelial cell growth by Kaposi’s sarcoma sera. J AIDS. 1990;3:677–82. [PubMed] [Google Scholar]
- 26.Del Papa N, Sheng YH, Raschi E, et al. Human β2-glycoprotein I binds to endothelial cells through a cluster of lysine residues that are critical for anionic phospholipid binding and offers epitopes for anti-β2-glycoprotein I antibodies. J Immunol. 1998;160:5572–8. [PubMed] [Google Scholar]
- 27.Ferraro G, Meroni PL, Del Papa N, et al. Anti-endothelial cell antibodies in patients with Wegener’s granulomatosis and micropolyarteritis. Clin Exp Immunol. 1990;79:47–53. doi: 10.1111/j.1365-2249.1990.tb05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Papa N, Raschi E, Catelli L, et al. Endothelial cells as a target for anti-phospholipid antibodies: role of anti-beta 2 glycoprotein I antibodies. Am J Repr Immunol. 1997;38:212–7. doi: 10.1111/j.1600-0897.1997.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 29.Carlos TM, Kovach NL, Schwartz BR, et al. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991;77:2266–71. [PubMed] [Google Scholar]
- 30.Beatty PG, Ledbetter JA, Martin PG, et al. Definition of a common leukocyte cell-surface antigen (Gp95-150) associated with diverse cell-mediated immune function. J Immunol. 1983;131:2913–8. [PubMed] [Google Scholar]
- 31.Sanchez Madrid F, Londazuri MO, Morago G, et al. VLA-3: a novel polypeptide association with the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986;16:1343–9. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzon P, Vecile E, Nardon E, et al. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J Cell Biol. 1998;142:1381–91. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francia di Celle P, Carbone A, Marchis D, et al. Cytokine gene expression in B-cell chronic lymphocytic leukemia: evidence of constitutive interleukin-8 (IL-8) expression and secretion of biologically active IL-8 protein. J Immunol. 1994;84:220–8. [PubMed] [Google Scholar]
- 34.Peri G, Milanese C, Matteucci C, et al. A new monoclonal antibody (5D3-F7) which recognizes human monocyte-chemotactic protein-1 but not related chemokines. Development of a sandwich ELISA and in situ detection of producing cells. J Immunol Methods. 1994;174:249–57. doi: 10.1016/0022-1759(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 35.Damel WW. Applied nonparametric statistics. Boston: Haughton Mifflin Co; 1978. [Google Scholar]
- 36.Biswas P, Delfanti F, Bernasconi S, et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in U937 cell line. Blood. 1998;91:258–65. [PubMed] [Google Scholar]
- 37.Haskill S, Johnson C, Eierman D, et al. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988;140:1690–4. [PubMed] [Google Scholar]
- 38.Kasahara K, Strieter R, Chensue T, et al. Mononuclear cell adherence induces neutrophil chemotactic factor/interleukin-8 gene expression. J Leuk Biol. 1991;50:287–95. doi: 10.1002/jlb.50.3.287. [DOI] [PubMed] [Google Scholar]
- 39.Sporn SA, Eierman DF, Johnson CE, et al. Monocyte adherence results in selective induction of novel genes shearing homology with mediators of inflammation and tissue repair. J Immunol. 1990;144:4434–41. [PubMed] [Google Scholar]
- 40.Newman S, Tucci M. Regulation of human monocyte/macrophage function by extracellular matrix. J Clin Invest. 1990;86:703–14. doi: 10.1172/JCI114766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlos T, Harlan J. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]
- 42.Ferreira OJ, Garcia PA, Bianco C. Specific binding of the human monocyte cell line U937 to the alternatively spliced connecting segment (III CS) of fibronectin. J Exp Med. 1990;171:351–6. doi: 10.1084/jem.171.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–18. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 44.Ho WZ, Li YH, Zu XH, et al. Induction of HIV-1 expression in chronically infected promonocytic cells co-cultured with human lung fibroblasts. Clin Immunol Immunopathol. 1996;80:171–8. doi: 10.1006/clin.1996.0111. [DOI] [PubMed] [Google Scholar]
- 45.Vitkovic L, Wood GP, Major EO, et al. Human astrocytes stimulate HIV-1 expression in a chronically infected promonocyte clone via interleukin-6. AIDS Res Hum Retrovir. 1991;7:723–7. doi: 10.1089/aid.1991.7.723. [DOI] [PubMed] [Google Scholar]
- 46.Vitkovic L, Kalebic K, de Cunha A, et al. Astrocyte-conditioned medium stimulates HIV-1 expression in a chronically infected promonocyte clone. J Neuroimmunol. 1990;30:153–60. doi: 10.1016/0165-5728(90)90099-9. [DOI] [PubMed] [Google Scholar]
- 47.Weiss L, Si-Mohamed A, Giral P, et al. Plasma levels of monocyte chemoattractant protein-1 but not those of macrophage inhibitory protein-1alpha and RANTES correlate with virus load in human immunodeficiency virus infection. J Infect Dis. 1997;176:1621–4. doi: 10.1086/517341. [DOI] [PubMed] [Google Scholar]
- 48.Bernasconi S, Cinque P, Peri G, et al. Selective elevation of monocyte chemotactic protein-1 (MCP-1) in the cerebrospinal fluid of AIDS patients with cytomegalovirus encephalitis. J Infect Dis. 1996;174:1098–101. doi: 10.1093/infdis/174.5.1098. [DOI] [PubMed] [Google Scholar]
- 49.Cinque P, Vago L, Dahl H, et al. Polymerase chain reaction on cerebro-spinal fluid for diagnosis of virus-associated opportunistic disease of central nervous system in HIV-infected patients. AIDS. 1996;10:951–8. doi: 10.1097/00002030-199610090-00004. [DOI] [PubMed] [Google Scholar]
- 50.Cinque P, Vago L, Mengozzi M, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–32. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Gong HJ, Ratkay LG, Waterfield JD, et al. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med. 1997;186:131–7. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]


