Abstract
A contraceptive vaccine directed against human chorionic gonadotropin (hCG) has previously undergone clinical testing that demonstrated the feasibility of the approach in preventing pregnancy in women. Some immunized volunteers however, did not respond with an adequate anti-hCG antibody response despite employing highly immunogenic bacterial toxoids as carriers. Since there is some evidence that T cell responses to a complex protein typically focus on a few immunodominant epitopes, we investigated the responsiveness to hCG in mice of different haplotypes using the protein carrier diphtheria toxoid (DT). Our data showed a differential carrier effect of DT. With the aim of making a more potent immunogen employing promiscuous pathogen-derived Th peptides as carriers, peptide:antigen stoichiometric ratios were optimized. When tested individually using alum as the adjuvant, three such peptide conjugates improved the anti-hCG response, though not consistently to levels higher than the DT conjugate. Immunization with a combination of these synthetic epitopes generated anti-hCG responses higher than those achieved with DT or with the individual peptides. Antibodies were of high affinity and capable of neutralizing the bioactivity of hCG, but were devoid of anti-peptide reactivity. These results support our view that differential hyporesponsiveness in a diverse population may arise from inadequate carrier effect and that it can be overcome by use of pathogen-derived broadly reactive non-B Th epitopes employing only alum, a permissible adjuvant.
Keywords: human chorionic gonadotropin, immunocontraception, Th peptide
INTRODUCTION
Linkage to a protein carrier has been used as a strategy to overcome immunological tolerance against human chorionic gonadotropin (hCG), a hormone that is produced by the peri-implantation embryo and plays a critical role in the establishment and maintenance of early pregnancy. The β-subunit of hCG (β hCG) conjugated chemically to diphtheria toxoid (DT) or tetanus toxoid (TT), induced anti-hCG antibody responses not only in animals but also in humans [1,2]. Carriers recruit Th cells that provide help to antibody-producing hCG-specific B cells. Initial human clinical studies with a β hCG-based contraceptive vaccine demonstrated the lack of notable side-effects [3], the generation of bioneutralizing antibodies [2,4] and the feasibility of the approach in preventing pregnancy in fertile women [5]. Protective levels of antibodies were however, not attained in some individuals. One reason for the diminished response may be related to carrier-mediated epitopic suppression [6–12], in which prior immunity against a carrier protein can inhibit the antibody response to a ligand attached to it. The phenomenon is however, not well established in humans [10].
It has been shown that the T cell response to a complex protein antigen usually focuses on a few, and frequently a single, immunodominant epitopes [13,14]. We therefore hypothesized that differential ability to respond to the carrier may be responsible for lower responses to hCG in some individuals of a diverse population. Given that carrier linkage is not only a critical strategy for contraceptive vaccines but is also essential for evoking antibody responses to subunit peptide vaccines, it becomes imperative to devise an alternative approach that can ensure generation of an adequate anti-ligand antibody response in most if not all recipients. In the present study, we investigated the feasibility of using promiscuous Th epitopes as carriers. pCST3 corresponds to residues 378–398 of the circumsporozoite protein of Plasmodium falciparum with cysteine residues 384 and 389 substituted by alanine residues [15] and an additional cysteine at the N-terminal, pMTB corresponds to residues 350–369 of the 38-kD protein of Mycobacterium tuberculosis[16] with an additional cysteine at the N-terminal, and pRSV represents residues 45–60 of the 1A protein of the respiratory syncytial virus [17]. These peptides were synthesized and individually linked to the heterospecies dimer (HSD, β hCG associated with α-ovine LH, β hCG-α oLH). Employing a permissible adjuvant, the conjugates were investigated individually as well as in combination for their immunogenic potential in inbred mice of various haplotypes.
MATERIALS AND METHODS
Design of immunogens
Amino acid sequences of the peptides used in the study are: pCST3 (CDIEKKIAKMEKASSVFNVVNS), pRSV (CEYNVFHNKTFELPRA) and pMTB (CDQVHFOPLPPAVVKLSDALI). The peptides were synthesized by a stepwise solid-phase procedure using the 430A peptide synthesizer (Applied Biosystems, Foster City, CA). Synthesis was carried out on a 0·5-mmol scale using phenylacetamidomethyl resin. The peptides were deprotected and cleaved from the resin using trifluoroacetic acid (TFA)-trifluoromethanesulphonic acid-thioanisole-ethanedithiol [18] and then purified by gel filtration on Sephadex G-25 in 10% acetic acid followed by reverse-phase high performance liquid chromatography (HPLC) on a C-18 column (Waters, Milford, MA). Aqueous TFA (0·1%; Solvent A) and 0·1% TFA in 80% aqueous acetonitrile (Solvent B) were used to form a linear gradient of 0–100% Solvent B in 35 min at a flow rate of 1·5 ml/min and A226 was monitored. Purity of the peptides, assessed by analytical reverse-phase HPLC and amino acid analysis, was >95%.
β hCG was associated with α oLH to create HSD following the procedure described earlier [19]. HSD was coupled to individual peptides using N-succinimidyl-3-(2-pyridyl dithio) propionate (SPDP; Pierce, Rockford, IL) [20]. Briefly, HSD (7 mg dissolved in 100 mm PBS pH 7·4) was treated at 25°C for 1 h with 10, 20 or 30 m excess of SPDP. Unreacted SPDP was removed by gel filtration on a Sephadex G-25 column. The numbers of lysine residues activated per HSD molecule were 3, 5 and 10, respectively. Activated HSD and pCST3 were mixed in a ratio of three peptide molecules for every 2-pyridyl-disulphide group and incubated for 2 h at 25°C and overnight at 4°C. By measuring the release of pyridine-2-thione at 343 nm, the number of pCST3 molecules conjugated to each HSD molecule was computed to be 2·5, 4 and 10, respectively. Conjugates with pRSV and pMTB (molar ratio ∼3:1) were similarly prepared. DT-HSD was prepared as described previously [21].
Immunization
Female 8–10-week-old BALB/c (H-2d), CBA (H-2k), C57Bl/6 (H-2b), FVB (H-2q) and SJL/Pas (H-2s) mice were used for the study. Groups of 8–10 mice each were immunized with antigens adsorbed on alum, Al(OH)3 (Superfos, Federikssund, Denmark). A total of three injections, at 4-week intervals, was given intramuscularly at two contralateral sites in a total volume of 100 μl. The mice were bled from the retro-orbital plexus. Sera were diluted appropriately and stored at −70°C until assayed.
Characterization of antibody response
Anti-hCG antibody titres expressed as hCG binding capacity were determined by a direct binding radioimmunoassay (RIA). hCG was iodinated (sp. act. 40–60 μCi/μg) by the Iodogen method [22]. The assay protocol consisted of 100 μl of diluted antiserum, 100 μl of 125I-hCG (approx. 15 000 dpm), 100 μl of 20% horse serum and 200 μl of assay buffer (50 mm PBS with 0·1% bovine serum albumin (BSA), pH 7·2). After incubation at 4°C for 20 h, the antibody-bound fraction was precipitated by adding 500 μl polyethylene glycol (PEG) 8000 (12·5% final concentration) and centrifugation at 1500 g for 20 min. The pellet was counted in a multi-γ-counter (LKB 1260; Turku, Finland). After correcting for non-specific binding, hCG binding capacity was calculated at a point(s) where proportionality was obtained between antiserum dilutions and labelled hCG binding. The hCG neutralization capacity of antibodies was measured by a receptor binding inhibition assay as described [5]. Association constant (Ka) of the antibody–hCG interaction was determined by cold displacement analysis described earlier [2].
Anti-DT or anti-peptide antibody titres were determined by standard ELISA systems. A 96-well plate was coated with DT or individual peptides (1 μg/well) in PBS pH 7·4 and non-specific sites saturated with 1% of low fat milk powder. After incubation with various serum dilutions, goat anti-mouse–horseradish peroxidase (HRP) conjugate was added as the revealing antibody. Orthophenylene diamine was employed as substrate. Antibody titres are expressed as antibody units, the product of A490 and antiserum dilution, and were calculated at points where decrease in absorbance was proportional to antiserum dilution.
Lymphocyte transformation assay
Mice were immunized in the hind footpads with peptide (100 μg) or DT (10 μg) in saline, emulsified in Freund's complete adjuvant (FCA). Ten days later, the draining popliteal and inguinal lymph nodes were removed. Single-cell suspensions were prepared. Cells (3 × 105/well) were cultured in 96-well plates in Dulbecco's modified Eagle's medium (DMEM; Gibco Labs, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Boehringer, Mannheim, Germany), 10 mm HEPES, 50 μm mercaptoethanol, 2 mm l-glutamine, 100 U/ml sodium benzyl penicillin, 100 μg/ml streptomycin sulphate and 3·7 g/l sodium bicarbonate. Increasing concentrations of the immunizing antigens were added in a total volume of 200 μl. All assays were performed in triplicate. For determining background proliferation, cells were cultured in medium only. After incubation at 37°C in a 5% CO2 atmosphere for 4 days, cells were pulsed with 0·5 μCi/50 μl of 3H-thymidine (Amersham, Aylesbury, UK) per well for 18 h. Cells were harvested on glassfibre filter mats (Pharmacia, Uppsala, Sweden). Incorporation of radioactive thymidine as an index of cell proliferation was estimated on a liquid scintillation counter (1205 Betaplate; LKB).
Statistical analysis
Statistical analysis of data was carried out on log transformed values of antibody titres. The significance level was calculated by Student's unpaired t-test, and considered significant if P < 0·05.
RESULTS
Carrier effect of DT in mice of different genetic backgrounds
To assess the general effectiveness of the carrier DT, mice of five different H-2 haplotypes (b, d, k, s, and q) were immunized with DT–HSD conjugate or HSD alone. Groups of 8–10 mice each received three injections of alum-adsorbed antigen at 4-week intervals. The animals were bled periodically and hCG binding capacity was measured in individual sera. Figure 1 shows the peak anti-hCG antibody titres, attained at 9 weeks after immunization. All groups immunized with the DT conjugate generated antibody responses consistently higher (P < 0·01) than those immunized with the unconjugated HSD. Comparison of the geometric means show that anti-hCG titres induced by the DT conjugate in CBA, C57Bl/6 and SJL mice were however, lower than the responses generated in BALB/c or FVB mice. Thus it is apparent that while DT induced enhancement of anti-hCG titres in all the strains tested, T cell help provided is strain-specific.
Fig. 1.
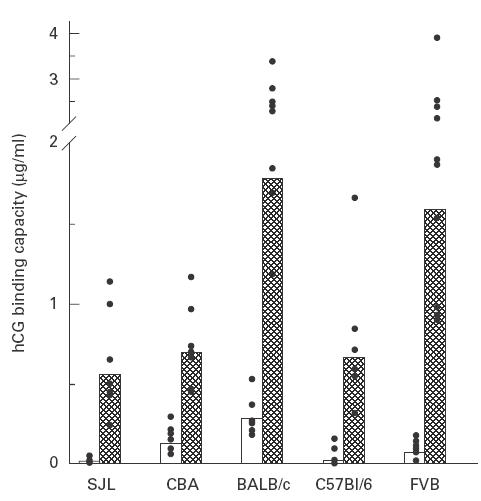
Immunogenic response to diphtheria toxoid (DT)–heterospecies dimer (HSD) in mice of different haplotypes. Groups of 8–10 mice were immunized with alum adsorbed DT–HSD (cross‐hatched) or HSD alone (□) by giving three injections at 4-week intervals. Peak antibody titres (week 9) in individual sera are shown as points and the bar represents geometric mean of the group.
Immunogenicity of pCST3 conjugates: effects of stoichiometry
The effect of conjugation of pCST3 on the immunogenicity of HSD was evaluated in mice of five different strains. Groups of mice were injected with HSD alone or pCST3–HSD conjugates of different stoichiometry (2·5, 4 or 10 peptides per HSD molecule). One group in each strain received HSD linked to DT (DT–HSD). The mice were bled at regular intervals and anti-hCG antibody titres were determined in individual sera. Results summarized in Fig. 2 indicate that conjugation of HSD to pCST3 significantly enhanced anti-hCG antibody responses in all the five mouse strains. For instance, all BALB/c mice receiving HSD–peptide conjugates responded with formation of anti-hCG antibodies, in contrast to only seven out of 10 mice immunized with HSD. Peak antibody titres induced by each of the pCST3 were significantly higher (P < 0·01) compared with mean responses in HSD-immunized mice. The potentiation in the immune response achieved by 2·5, 4 or 10 copies of pCST3 was not significantly different (geometric mean hCG binding capacity 2619, 1646, 1966 ng/ml for respective pCST3 conjugates versus 152 ng/ml for HSD) and was comparable to that achieved with DT (1922 ng/ml). Although the overall levels of antibody responses were lower, mean anti-hCG titres in SJL/Pas (304, 273, 497 ng/ml), CBA (449, 331, 434 ng/ml) and C57Bl/6 mice (337, 529, 592 ng/ml) were significantly higher (P < 0·01) when compared with responses in groups immunized with HSD alone (67, 35 and 52 ng/ml, respectively). In FVB mice, the conjugate pCST3–HSD (2·5:1 molar ratio) substantially elevated (P < 0·01) the mean antibody titres (1108 ng/ml) over those attainable with unconjugated HSD (47 ng/ml), the improvement being of the same order as achieved with DT conjugation (1050 ng/ml). Increasing peptide loading (4 or 10) appeared to enhance the response further (1544 and 1694 ng/ml, respectively); the differences were however, not statistically significant. Since an epitopic density of 2·5, 4 and 10 peptides per HSD molecule gave essentially similar antibody responses, a stoichiometry of about three Th epitopes per HSD molecule was used for further studies.
Fig. 2.
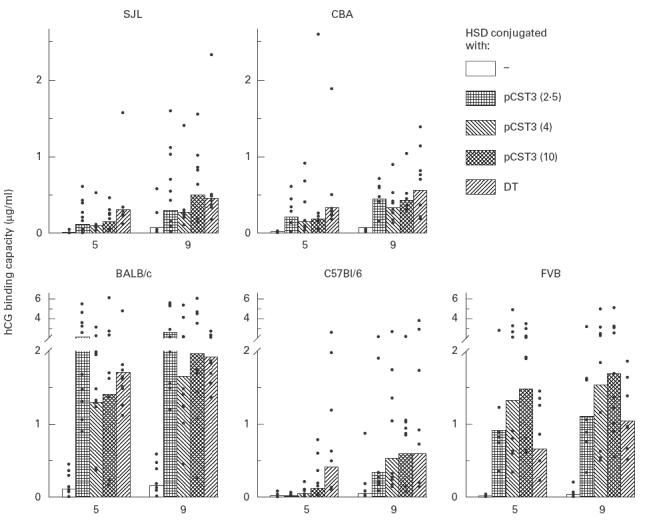
Effect of pCST3 conjugation on immunogenicity of heterospecies dimer (HSD). Groups of mice in each strain received pCST3–HSD conjugates (molar ratio 2·5:1, 4:1 and 10:1), HSD alone or diphtheria toxoid (DT)–HSD. Three injections of alum-adsorbed antigens (5 μg gonadotropin equivalent) were given intramuscularly at 0, 4 and 8 weeks. Antibody titres at 5 and 9 weeks are shown in individual animals as points and the bar represents the geometric mean of the group. pCST3 > HSD (P < 0·01) all groups, pCST3 versus DT (NS) all groups.
Immunogenicity of conjugates with pMTB and pRSV
In the next set of experiments, pRSV and pMTB were investigated for their ability to provide T cell help to HSD. Each peptide was individually linked to HSD and immunogenic potential of the conjugated antigens tested. Controls included mice immunized with HSD alone or with DT–HSD. Analysis of anti-hCG responses shown in Fig. 3 indicated that conjugation with pMTB and pRSV significantly improved the immunogenicity of HSD in four of the five mouse strains tested (BALB/c, 281 ng/ml versus 1789 and 1843 ng/ml (P < 0·01), respectively; FVB, 89 versus 570 and 1715 (P < 0·01); CBA, 158 versus 327 and 418 (P < 0·05); SJL, 14 versus 200 and 300 (P < 0·01). While the two peptide conjugates were as immunogenic as the DT conjugate in BALB/c mice (1888 ng/ml), in FVB and CBA strains only pRSV–HSD generated antibody titres comparable to those generated by DT–HSD (1718 and 699 ng/ml, respectively). In C57Bl/6 mice only pMTB was found to be active, with mean antibody titres being 625 ng/ml (versus 23 ng/ml for HSD; P < 0·01), comparable to those generated by DT–HSD (666 ng/ml).
Fig. 3.
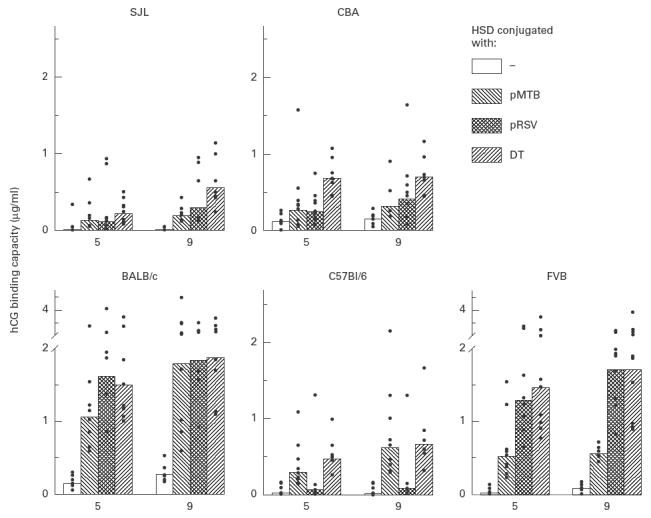
Carrier effect of pMTB and pRSV in different mouse strains. Mice (8–10 per group) were immunized with pMTB–heterospecies dimer (HSD), pRSV–HSD, diphtheria toxoid (DT)–HSD or HSD alone. In all, three injections of alum-adsorbed antigen were given at an interval of 4 weeks. Antibody titres at 5 and 9 weeks are shown in individual animals as points and the bar represents the geometric mean of the group. Statistical analysis at week 9: BALB/c-pRSV–HSD/pMTB–HSD, versus HSD (P < 0·01), versus DT–HSD (NS); C57Bl/6-pMTB–HSD, versus HSD (P < 0·01), versus DT–HSD (NS); FVB-pRSV–HSD/pMTB–HSD, versus HSD (P < 0·01); pRSV–HSD versus DT–HSD (NS); SJL-pRSV–HSD/pMTB–HSD versus HSD (P < 0·01); pRSV–HSD versus DT–HSD (NS); CBA-pRSV–HSD versus HSD (P < 0·01), versus DT–HSD (NS); pMTB–HSD versus HSD (P < 0·05).
Efficacy of a combination of peptides conjugates
We next tested whether a combination of the three peptide conjugates would lead to an additive effect in enhancing the antibody responses and help overcome the genetic hyporesponsiveness of the protein carrier. An equimolar physical mixture of peptide conjugates adsorbed on alum was administered to groups of mice as a composite preparation. Results shown in Fig. 4 reveal that each of the five different mouse strains responded with an elevated anti-hCG antibody response, clearly establishing the advantage of the mixture over individual peptide conjugates. While antibody titres generated by the peptide mixture were significantly higher (P < 0·05) than those elicited by the DT-conjugated antigen in BALB/c (2316 versus 1472 ng/ml), FVB (3791 versus 1849 ng/ml), CBA (1734 versus 1025 ng/ml) and C57Bl/6 mice (985 versus 505 ng/ml), the response in SJL mice was similar to that observed in the group immunized with DT–HSD (1198 versus 1151 ng/ml). When the data were analysed collectively, the antibody response of all five strains (akin to studying the immune response in heterogeneous population) with peptide mixture-based formulation (geometric mean 1800 ng/ml) was found to be statistically higher (P < 0·01) than the responses of all DT-immunized groups (1105 ng/ml). Antibodies were found competent to inhibit the bioactivity of hCG in vitro. Our previous studies have established a good correlation between the contraceptive efficacy of the vaccine and the in vitro neutralization capacity of the antibodies [5]. Although the bioneutralization capacity per ml serum was consistently higher for all peptide groups when compared with the capacity of corresponding DT groups (Fig. 5), the difference for SJL mice was however, not significant. Antibodies generated by the cocktail HSD–peptide conjugates in all strains had high affinity for hCG; association constants (Ka), computed by Scatchard analysis, were of the order of 1010 M−1.
Fig. 4.
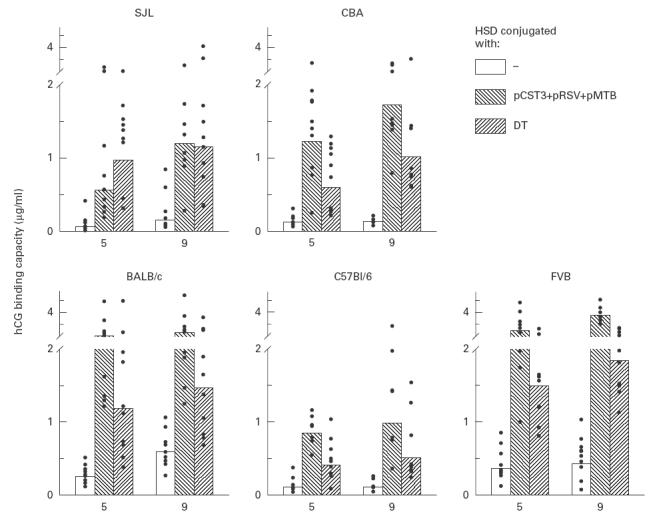
Enhanced immunogenicity of a cocktail mixture of peptide conjugates. Mice were immunized with a physical mixture of the pCST3–heterospecies dimer (HSD), pMTB–HSD and pRSV–HSD conjugates adsorbed on alum. Three injections each containing an equimolar mixture of conjugates (5 μg each) were given intramuscularly at an interval of 4 weeks. Groups of mice were also immunized with HSD alone or diphtheria toxoid (DT)–HSD. Antibody titres at 5 and 9 weeks are shown in individual sera as points with bar as geometric mean of the group. Mean peak titres with peptide mixture is significantly higher (P < 0·05) than corresponding DT–HSD groups of all strains except SJL.
Fig. 5.
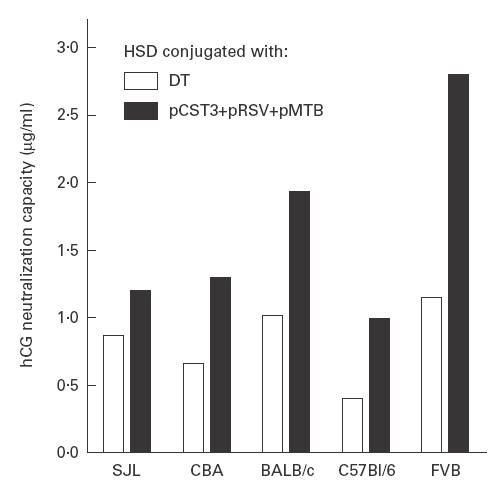
Human chorionic gonadotropin (hCG) bioneutralization efficacy of the antibodies elicited by peptide mixture. Capacity to neutralize hCG bioactivity of week 9 bleeds was measured by a receptor binding inhibition assay. Neutralization capacity per ml serum is higher in all the peptide groups than in the corresponding diphtheria toxoid (DT) groups, the differences being statistically significant (P < 0·05) in BALB/c, CBA, C57Bl/6 and FVB.
Anti-DT and anti-peptide antibody responses
Preimmune sera did not exhibit titres to any of the three peptides or to DT. As expected, animals immunized with the DT–HSD conjugate responded by generating an anti-DT antibody response with titres ranging from 27 to 437 antibody units (the product of A490 and antiserum dilution). Significantly, antibodies were not detectable (< 1 U) to any of the three peptides in animals immunized with the peptide cocktail.
In vitro lymphocyte proliferation
Lymphocyte transformation assays were performed with the synthetic peptides to assess their potential to induce T cell proliferation in vitro. Mice were immunized with individual peptides or with DT and recall responses were assayed against respective antigens. The data show strain- and dose-dependent lymphocyte proliferative responses (Fig. 6). In SJL/Pas (H-2s) mice, all three peptides tested induced appreciable proliferation of lymph node cells, with pRSV inducing the highest proliferative response. FVB (H-2q) and CBA (H-2k) mice responded better to the pRSV than to the other peptides; in BALB/c (H-2d) mice, pCST3 and pRSV induced a greater degree of proliferation compared with pMTB. C57Bl/6 (H-2b) mice responded well to pMTB and pRSV, but proliferation to pCST3 was low at all doses tested.
Fig. 6.
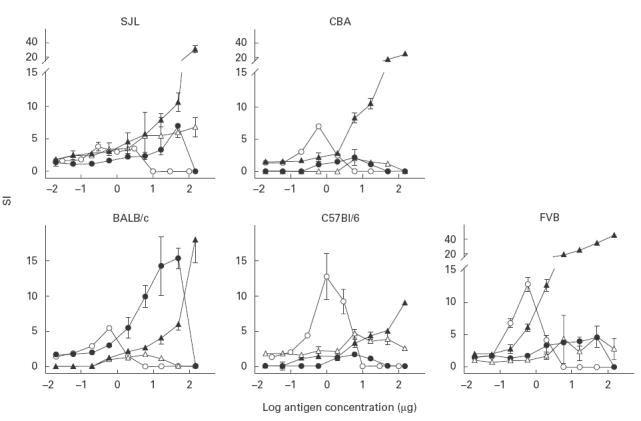
Lymphoproliferative responses in mice immunized with pCST3 (•), pMTB (Δ), pRSV (▴) and diphtheria toxoid (DT) (○). Animals were immunized with the respective antigen after emulsifying in Freund's complete adjuvant (FCA). Ten days later, single-cell suspensions were prepared from lymph nodes and incubated with a range of concentrations of the immunizing antigen. The recall responses to the peptides or to DT were determined by in vitro3H-thymidine uptake and are expressed as stimulation indices (SI, the ratio of the mean counts in the wells with and without antigen).
DISCUSSION
In the present study, we demonstrate that hyporesponsiveness to the conjugate hCG vaccine is related to selective deficiency of adequate carrier effect by protein antigens in a genetically diverse population. This hyporesponsiveness can be overcome by using an appropriate combination of pathogen-derived promiscuous non-B Th epitopes. Mice immunized with a protein carrier exhibit strain-specific variation in anti-hCG response. Since these animals had no prior immunity against the carrier DT, the lower response observed in three strains cannot be attributed to carrier-induced epitopic suppression, a phenomenon observed in a variety of hapten-carrier systems [6–12]. Epitopic suppression has been attributed to the presence of anti-carrier antibodies [10], to induction and activation of suppressor T cells [7,8–10] or to antigenic competition between carrier-specific and ligand-specific B cells [9]. Since β hCG is poorly immunogenic by itself, it can be concluded that even a large carrier such as DT provides differential carrier effect (T cell help). A lower responsiveness to the carrier might lead to inadequate generation of T cell help, which in turn would lead to a lower anti-hCG antibody response in some recipients. This could pose problems when an outbred population is to be vaccinated. A diminished response in some women [5] might be attributed to HLA haplotype distribution.
We evaluated the potential of three promiscuous Th peptides with a view to using them as substitute carriers for a large carrier protein. CST3 (p378–398 of the CS protein of P. falciparum) has been reported to be recognized by a large number of mouse and human MHC class II molecules and to induce an antibody response to the conjugated B cell epitope (NANP)3 in mice of different strains, using FCA as the adjuvant [15]. Our studies demonstrate the potential of pCST3 to provide T cell help for in vivo antibody production against a covalently linked antigen (HSD) without the use of FCA. HSD being ‘foreign’, mice do respond by producing anti-hCG antibodies, albeit of low order, when it is injected after adsorption on alum. If however, the molecule is linked to pCST3, anti-hCG response increases several-fold. The strain-specific response pattern is similar to anti-NANP responses obtained when CST3 was linked with the B cell epitope (NANP)3[15], indicating that the nature of the conjugated B cell antigen is probably not an important factor in determining the haplotype-dependent hierarchy of antibody responses. Our data show that pMTB and pRSV also consistently improved the immune response when conjugated to HSD, suggesting that adequate processing and presentation of Th peptides occur even when the peptide is linked to HSD, a 38-kD protein. In our studies, there appears to be no advantage in increasing stoichiometry beyond 2·5 peptides per HSD molecule. The importance of optimizing stoichiometry has been described in other systems. Tam et al. [23] reported that multiple antigen peptides (MAPs) with four or eight tandemly linked B and T cell epitopes of P. berghei CS protein were highly immunogenic, while those containing B and T cell epitopes in a ratio of 8:1 or 1:8 were not as immunogenic. Similarly, heat-stable enterotoxin of Escherichia coli has been shown to be more immunogenic when linked to four copies of a Th cell epitope from ovalbumin (p323–329) than when attached to a single or two copies of the peptide; a construct with a single peptide required a 20-fold higher dose for a response similar to that with four copies of the Th epitope [24]. We found no correlation between lymphoproliferative responses induced by the peptides in vitro and anti-hCG antibodies generated by respective HSD–peptide conjugates. Similar lack of correlation has been observed for a malarial antigen [25]. This is not entirely unexpected, given the different routes of immunization and the adjuvants used.
Although the three epitopes we employed are promiscuous, i.e. they are recognized in the context of a variety of MHC/HLA alleles, they were not found equally reactive with different alleles. A given peptide may not be able to bind to all MHC alleles in the same orientation or with the same sequence [15,17], resulting in variable affinity to MHC and presentation to T cells. Interaction between MHC–peptide–TCR may significantly influence the induction of humoral responses. We reasoned that the use of a combination of Th peptides instead of a large carrier might help overcome this limitation of genetic restriction for production of anti-hCG antibodies. Our data show that co-injecting an equimolar mixture of three peptide conjugates led to a significant enhancement of the immune responses in five different strains of mice. Antibodies were bioneutralizing in nature, indicating that peptide linkage did not affect crucial B cell epitopes.
While earlier studies demonstrating improved immunogenicity with Th epitope conjugation employed FCA [26–33], we employed only alum, an adjuvant approved for human use. It is possible that immunization with the cocktail leads to the recruitment of a larger repertoire of T cells. It has been shown that the simultaneous presence of immunodominant T cell epitopes does not result in competition when the peptides are injected together [34]. Such co-dominance is anticipated among peptides that bind with relatively high affinity to the presenting molecules and thus have a chance to occupy a significant number of binding sites for T cell activation. An additive synergism, where the presence of a peptide enhances the response to another, is reported [34].
The peptides investigated here contain information for helper function and do not induce antibodies against themselves. Responses elicited by the peptide conjugates should not be susceptible to carrier-specific B and Ts cell-mediated suppression. In fact, for peptide conjugate vaccines, prior natural exposure to the infectious agents from which the peptides are derived may prove to be beneficial rather than detrimental. TT-primed mice, when immunized with the B cell epitope (NANP)4 linked to a Th peptide from TT, generated an antibody response to (NANP)4. The response was greater than when they received the B cell epitope linked to TT [11].
In conclusion, our data show that hyporesponsiveness to a conjugate vaccine may result from deficient carrier effect and can be bypassed by using a combination of promiscuous Th cell peptides as a substitute for protein carriers. Absence of B and Ts epitopes in these sequences would eliminate the possibility of carrier-induced epitopic suppression in recipients having prior immunity against carrier. These findings have implications for the development of contraceptive vaccines in particular and subunit vaccines in general, which aim at inducing protective levels of antibody titres in a majority of individuals of a diverse population.
Acknowledgments
This work was supported by grants from the Department of Biotechnology, Government of India, and the International Development Research Centre (IDRC) of Canada.
REFERENCES
- 1.Talwar GP, Sharma NC, Dubey SK, Salahuddin M, Das C, Ramakrishnan S, Kumar S, Hingorani V. Isoimmunization against human chorionic gonadotropin with conjugates of processed β-subunit of the hormone and tetanus toxoid. Proc Natl Acad Sci USA. 1976;73:218–22. doi: 10.1073/pnas.73.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh Om, Rao LV, Gaur A, Sharma NC, Alam A, Talwar GP. Antibody response and characteristics of antibodies in women immunized with three contraceptive vaccines inducing antibodies against human chorionic gonadotropin. Fertil Steril. 1989;52:739–44. [PubMed] [Google Scholar]
- 3.Talwar GP, Hingorani V, Kumar S, et al. Phase I clinical trials with three formulations of anti-human chorionic gonadotropin vaccine. Contraception. 1990;41:301–16. doi: 10.1016/0010-7824(90)90071-3. [DOI] [PubMed] [Google Scholar]
- 4.Pal R, Singh Om, Rao LV, Talwar GP. Bioneutralization capacity of the antibodies generated in women by the beta subunit of human chorionic gonadotropin (βhCG) and βhCG associated with alpha subunit of ovine luteinizing hormone linked to carriers. Am J Reprod Immunol. 1990;22:124–6. doi: 10.1111/j.1600-0897.1990.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 5.Talwar GP, Singh Om, Pal R, et al. A vaccine that prevents pregnancy in women. Proc Natl Acad Sci USA. 1994;91:8532–6. doi: 10.1073/pnas.91.18.8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzenberg LA, Tokuhisa T, Herzenberg LA. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664–7. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 7.Herzenberg LA, Tokuhisa T. Epitope-specific regulation: I. Carrier-specific induction of suppression for IgG anti-hapten antibody responses. J Exp Med. 1982;155:1730–40. doi: 10.1084/jem.155.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schutze MP, Leclerc C, Vogel FR, Chedid L. Epitopic suppression in synthetic vaccine models: analysis of effector mechanisms. Cell Immunol. 1987;104:79–90. doi: 10.1016/0008-8749(87)90008-6. [DOI] [PubMed] [Google Scholar]
- 9.Schutze MP, Deriaud E, Przewlocki G, Lecrec C. Carrier-induced epitopic suppression is initiated through clonal dominance. J Immunol. 1989;142:2635–40. [PubMed] [Google Scholar]
- 10.Di John D, Wasserman SS, Torres JR, et al. Effect of priming with carrier on response to conjugate vaccine. Lancet. 1989;2:1415–8. doi: 10.1016/s0140-6736(89)92033-3. [DOI] [PubMed] [Google Scholar]
- 11.Etlinger HM, Gillessen D, Lahm HW, Matile H, Schonfeld HJ, Trzeciak A. Use of prior vaccinations for the development of new vaccines. Science. 1990;249:423–5. doi: 10.1126/science.1696030. [DOI] [PubMed] [Google Scholar]
- 12.Renjifo X, Wolf S, Pastoret PP, Bazin H, Urbain J, Leo O, Moser M. Carrier-induced, hapten-specific suppression: a problem of antigen presentation? J Immunol. 1998;161:702–6. [PubMed] [Google Scholar]
- 13.Katz ME, Maizels RM, Wicker L, Miller A, Sercarz EE. Immunological focusing by the mouse major histocompatibility complex: mouse strains confronted with distantly related lysozymes confine their attention to very few epitopes. Eur J Immunol. 1982;12:535–40. doi: 10.1002/eji.1830120702. [DOI] [PubMed] [Google Scholar]
- 14.Berkower I, Matis LA, Buckenmeyer GK, Gurd FR, Longo DL, Berzofsky JA. Identification of distinct predominant epitopes recognized by myoglobin-specific T cells under the control of different Ir genes and characterization of representative T cell clones. J Immunol. 1984;132:1370–8. [PubMed] [Google Scholar]
- 15.Sinigaglia F, Guttinger M, Kilgus J, et al. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988;336:778–80. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- 16.Vordermeier HM, Harris DP, Roman E, Lathigra R, Moreno C, Ivanyi J. Identification of T cell stimulatory peptides from the 38-kDa protein of Mycobacterium tuberculosis. J Immunol. 1991;147:1023–9. [PubMed] [Google Scholar]
- 17.Nicholas JA, Levely ME, Mitchell MA, Smith CW. A 16-amino acid peptide of respiratory syncytial virus 1A protein contains two overlapping T cell-stimulating sites distinguishable by class II MHC restriction elements. J Immunol. 1989;143:2790–6. [PubMed] [Google Scholar]
- 18.Stewart JM, Young JD. Solid phase peptide synthesis. 2. Rockford: Pierce Chemical Co.; 1984. [Google Scholar]
- 19.Talwar GP, Singh Om, Rao LV. An improved immunogen for anti-human chorionic gonadotropin vaccine eliciting antibodies reactive with a conformation native to the hormone without cross-reaction with human follicle stimulating hormone and human thyroid stimulating hormone. J Reprod Immunol. 1988;14:203–12. doi: 10.1016/0165-0378(88)90020-4. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson J, Drevin H, Axen R. Protein thiolation and reversible protein–protein conjugation N-succinimidyl 3-(2-pyridyldithio) propionate, a new heterobifunctional reagent. Biochem J. 1978;173:723–37. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaur A, Arunan K, Singh Om, Talwar GP. Bypass by an alternate ‘carrier’ of acquired unresponsiveness to hCG upon repeated immunization with tetanus conjugated vaccines. Int Immunol. 1990;2:151–6. doi: 10.1093/intimm/2.2.151. [DOI] [PubMed] [Google Scholar]
- 22.Fraker PJ, Speck JC. Protein and cell membrane iodination with a sparingly soluble chloramide 1,3,4,6-tetrachloro-3α-6α-diphenylglycouril. Biochem Biophys Res Commun. 1978;80:849–55. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 23.Tam JP, Clavijo P, Lu YA, Nussenzweig V, Zavala F. Incorporation of T and B epitopes of the circumsporozoite protein in a chemically defined synthetic vaccine against malaria. J Exp Med. 1990;171:299–306. doi: 10.1084/jem.171.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowenadler B, Lycke N. Fusion proteins with heterologous T helper epitopes. Recombinant E coli heat stable enterotoxin proteins. Int Rev Immunol. 1994;11:103–11. doi: 10.3109/08830189409061718. [DOI] [PubMed] [Google Scholar]
- 25.Herrera S, De Plata C, Gonzalez M, Perlaza BL, Bettens F, Corradin G, Arevalo-Herrera M. Antigenicity and immunogenicity of multiple antigen peptides (MAP) containing P. vivax CS epitopes in Aotus monkeys. Parasite Immunol. 1997;19:161–70. doi: 10.1046/j.1365-3024.1997.d01-193.x. [DOI] [PubMed] [Google Scholar]
- 26.Good MF, Maloy WF, Lunde MN, et al. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987;235:1059–62. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- 27.Francis MJ, Hastings GZ, Syred AD, McGinn B, Brown F, Rowlands DJ. Non-responsiveness to a foot-and-mouth disease virus peptide overcome by addition of foreign helper T-cell determinants. Nature. 1987;330:168–70. doi: 10.1038/330168a0. [DOI] [PubMed] [Google Scholar]
- 28.Lecrec C, Przewlocki G, Schutze MP, Chedid L. A synthetic vaccine constructed by copolymerization of B and T cell determinants. Eur J Immunol. 1987;17:269–73. doi: 10.1002/eji.1830170218. [DOI] [PubMed] [Google Scholar]
- 29.Milich DR, Hughes JL, McLachlan A, Thornton GB, Moriarty A. Hepatitis B synthetic immunogen comprised of nucleocapsid T-cell sites and an envelope B-cell epitope. Proc Natl Acad Sci USA. 1988;85:1610–4. doi: 10.1073/pnas.85.5.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tindle RW, Fernando GJP, Sterling JC, Frazer IH. A ‘public’ T-helper epitope of E7 transforming protein of human papillomavirus 16 provides cognate help for several E7 B-cell epitopes from cervical cancer-associated human papillomavirus genotypes. Proc Natl Acad Sci USA. 1991;88:5887–91. doi: 10.1073/pnas.88.13.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Arora R, Kaur P, Chauhan VS, Sharma P. ‘Universal’ T helper cell determinants enhance the immunogenicity of a Plasmodium falciparum merozoite surface antigen peptide. J Immunol. 1992;148:1499–505. [PubMed] [Google Scholar]
- 32.Chengalvala MV, Bhat RA, Bhat BM, Vernon SK, Lubeck MD. Enhanced immunogenicity of hepatitis B surface antigen by insertion of a helper T cell epitope from tetanus toxoid. Vaccine. 1999;17:1035–41. doi: 10.1016/s0264-410x(98)00318-1. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh S, Jackson DC. Antigenic and immunogenic properties of totally synthetic peptide-based anti-fertility vaccines. Int Immunol. 1999;11:1103–10. doi: 10.1093/intimm/11.7.1103. [DOI] [PubMed] [Google Scholar]
- 34.Adorini LS, Appella E, Doria G, Nagy ZA. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988;168:2091–104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]


