Abstract
The aim of this study was to investigate the influence of IL-6 on mortality, bacterial growth and cytokine expression in experimental acute pyelonephritis. Female IL-6-deficient mice and their wild-type counterparts, 8–10 weeks old, were infected with Escherichia coli CFT 073 or injected with NaCl 0·9% (w/v) via the urethra and thereafter obstructed for 6 h. Animals were killed at 48 h, 6 days or 8 weeks and cytokine and bacterial renal levels were assessed at each time point. We found that IL-6-deficient mice had increased mortality and extensive renal bacterial growth on day 6, compared with wild-type mice (P < 0·05) and the histopathological changes were generally more severe and widespread in the IL-6-deficient mice. Peak mRNA expression of IL-1β, IL-4, IL-10, IL-12 and interferon-gamma (IFN-γ) occurred 48 h after infection in both IL-6 knock out and wild-type mice. Transforming growth factor-beta (TGF-β) levels also peaked at 48 h in E. coli-infected wild-type mice, while in the IL-6-deficient strain both TGF-β mRNA and protein levels were significantly lower at 48 h than wild-type levels (P < 0·0008 and P < 0·03, respectively) and remained stationary throughout the study period. Animals injected with NaCl 0·9% (w/v) displayed a similar decrease in TGF-β expression (P < 0·02). When splenocytes from the IL-6-deficient mice were incubated with murine recombinant IL-6, TGF-β levels increased to those of wild-type mice. No increase was observed when splenocytes from wild-type mice were incubated with the same doses of rIL-6. We therefore conclude that IL-6 plays an important role in bacterial clearance and directly influences the TGF-β levels in experimental acute pyelonephritis. We also demonstrate that urethral obstruction per se induces an increase in TGF-β the magnitude of which is decreased in IL-6-deficient mice.
Keywords: IL-6 knock out mice, acute pyelonephritis, Escherichia coli, transforming growth factor-beta
Introduction
IL-6 is a pleiotropic cytokine that plays an important role in host defence mechanisms, regulating both inflammatory responses and immune functions. It has a major effect on the production of acute-phase proteins and haematopoiesis [1], differentiation of T cells [2], activation of macrophages [3], and differentiation of B cells into antibody-producing plasma cells [4]. The IL-6 signal is transduced through a specific IL-6 receptor and a transmembrane protein, gp130, which is also involved in the signal transduction of other molecules such as leukaemia inhibitory factor, oncostatin M, ciliary neutropic factor and IL-11, all of which display overlapping activities [5, 6].
When bacteria adhere to uroepithelial cells an inflammatory response is provoked through endotoxin and various virulence factors. A high and rapid IL-6 response in the urine is induced by Escherichia coli strains producing virulence factors, such as cytotoxic necrotizing factor and haemolysin, compared with low-virulence strains [7]. In the kidneys, IL-6 production is induced within a few hours of the onset of experimental acute pyelonephritis and production remains increased for several days [8–11]. The early and maintained secretion of IL-6 in the kidneys is claimed to have merely proinflammatory effects [11]; however, both local and systemic anti-inflammatory effects of IL-6 have been reported, which were attributed to its regulatory effects on proinflammatory cytokines [12,13]. These findings raised the possibility that IL-6 plays a role in the pathogenesis of kidney disease [11]. In line with this hypothesis we found that only children with pyelonephritis and detectable IL-6 in their urine during the acute phase of disease were at risk of developing renal scarring [14].
IL-6-deficient mice carrying a null mutation in the IL-6 gene [15] represent a powerful tool to characterize unambiguously the role of IL-6 in acute pyelonephritis. In the present study we therefore investigated its significance in experimentally induced acute E. coli pyelonephritis in relation to mortality, bacterial growth in the kidneys and cytokine production, as well as the impact of urethral obstruction per se on the cytokine profile.
Materials and methods
Animals
IL-6-deficient female mice (n = 78) and their wild-type counterparts (n = 65) (8–10 weeks old, about 30 g) were used in this study. The generation of mice on a B6CBA background has been described before [15]. Four animals were housed in each cage, with food and water ad libitum.
Bacteria and preparation of inoculum
Escherichia coli CFT 073 was obtained from the blood of a patient with acute pyelonephritis (kindly provided by D. E. Johnson, VA Medical Center, Baltimore, MD). It expresses P, S and type 1 fimbriae, haemolysin and induces pyelonephritis in experimentally challenged normal mice. The bacteria were grown overnight on CLED agar at 37°C. A centrifuged cell pellet from this culture was resuspended in PBS, to give a final bacterial concentration of 109 colony-forming units (CFU)/ml.
Experimental pyelonephritis
Female IL-6-deficient mice and their wild-type counterparts were each first divided into two groups. One subgroup was infected with a bacterial suspension of E. coli CFT 073 (n = 24) and the other subgroup, serving as a control group, was inoculated with 0·9% NaCl (w/v) (n = 12). Animals were then killed at 48 h, 6 days or 8 weeks. Untouched animals, i.e. mice that were inoculated neither with E. coli nor with NaCl (n = 3 for both IL-6 knock out and wild-type mice), were killed at 0 h and also served as controls. In all other respects the animals were treated identically. Inoculation was performed by urethral catheterization as previously described [9] under anaesthesia with Diazepam 30 μl/30 g and Hypnorm 10 μl/30 g given intraperitoneally. Bacterial suspension (50 μl) or 0·9% NaCl (w/v) was deposited in the bladder through a soft sterile polyethylene catheter (outer diameter 0·61 mm and inner diameter 0·28 mm) (Clay Adams, Becton Dickinson, Pareippany, NJ). Immediately after the catheter was withdrawn, an obstruction with collodion (Sves 46; Swedish Pharmacopoeia) was applied for 6 h. India ink was added to monitor the integrity of the collodion and to enable detection of urine leakage on absorbent paper. Each mouse was housed separately during the first 6 h. All mice were killed at preset time points. Kidneys were removed aseptically and placed on dry ice and isopentone and stored at −70°C until analysed. Spleens were placed in cold Iscove's modified Dulbecco's medium (Flow Labs, Irvine, UK). Survival was monitored during the first 6 h and then daily.
Tissue processing for histopathology, in situ hybridization, enzyme immunosorbent assay and bacterial culture
The kidneys were cut into 1·1-mm slices using a device with parallel razor blades. Every second tissue slice was systematically and randomly sampled into two separate tissue blocks for histological evaluation and in situ hybridization, respectively. The latter were snap-frozen and cut in 10 μm thick sections in a cryostat. The frozen sections were placed on ProbeOn microscope slides (Fisher, Biotech, Pittsburgh, PA), and stored at −20°C until assayed. For histological evaluation, the tissue blocks were embedded in paraffin after fixation in 4% buffered paraformaldehyde. From these tissue blocks, 4 μm thick sections were cut and stained with haematoxylin and eosin. The inflammatory changes in each kidney were assessed using semiquantitative scoring (0–3): 0 = no inflammatory changes; 1 = pelvic inflammation; 2 = parenchymal inflammation adjacent to the fornices; 3 = inflammation involving medulla and cortex. The mean score was calculated for each group of animals. The sections were evaluated by the same investigator (G.J.) without knowledge of other laboratory data. For enzyme immunosorbent assay (EIA) and bacterial cultures, kidneys were homogenized at a ratio of 50 mg kidney/ml PBS. The homogenate was centrifuged at 300 g for 10 min and the supernatant was stored at −70°C until EIA assay was performed.
Preparation of mononuclear cell suspension
Each spleen was gently crushed through a stainless steel meshwork. The cells released were washed once in Iscove's modified Dulbecco's medium (Flow Labs). Erythrocytes in the resultant cell pellet were lysed by adding 2 ml cold sterile water for 30 s, followed by the addition of 2 ml 2·7% (w/v) NaCl. The splenocytes were then washed twice in medium and rediluted to obtain a cell concentration of 5 × 106/ml.
Bacterial growths
Homogenized kidneys were spread on blood and CLED agar and 10 μl of homogenate were also added to tryptic soy broth. Agar plates were incubated at 37°C for 48 h and the broth for 7 days. Numbers of colonies were counted on blood agar. Identification of E. coli was performed using standard criteria.
Detection of cytokine mRNA by in situ hybridization
In situ hybridization was performed as described previously [16]. Synthetic oligonucleotide probes (Scandinavian Gene Synthesis AB, Köping, Sweden) were labelled using 35S deoxyadenosine-5-α- (thio)-triphosphate (New England Nuclear, Cambridge, MA) with terminal deoxynucleotide transferase (Amersham, Aylesbury, UK). To increase the sensitivity of the method a mixture of four different oligonucleotide probes, approximately 48 base pairs long, were used. The oligonucleotide sequences for IL-1β, IL-4, IL-6, IL-10, IL-12, transforming growth factor-beta (TGF-β) and IFN-gamma (IFN-γ) were obtained from GenBank and probes were designed using Mac Vector software (Table 1). Probes complementary to antisense strands were used in parallel as controls to ensure the specificity of the hybridization signals. These control probes produced a uniformly weak background without revealing any positive cells. Emulsion autoradiography was performed and the slides were coded. Cells expressing more than 15 grains over the cytoplasm were counted by dark field microscopy at ×100 magnification. Accuracy was checked at a higher magnification and by light microscopy (Fig. 1). The results for each cytokine were expressed as the number of labelled positive cells per 100 mm2 tissue section. Tissue sections were measured by image analysis (Image Analysis Systems; Seescan, Cambridge, UK).
Table 1.
Probes used for detection of cytokine mRNA expression by in situ hybridization
| Probe | Gene bank acc number | Bases | |
|---|---|---|---|
| IL-1β | M98820 | 639–686 | (exon 1) |
| 569–616 | (exon 2) | ||
| 278–325 | (exon 3) | ||
| 295–342 | (exon 4) | ||
| IL-4 | X16058 | 83–130 | (exon 1) |
| 209–256 | (exon 2) | ||
| 270–317 | (exon 3) | ||
| 331–378 | (exon 4) | ||
| IL-6 | M26744 | 139–187 | (exon 1) |
| 180–223 | (exon 2) | ||
| IL-10 | M37897 | 79–126 | (exon 1) |
| 134–181 | (exon 2) | ||
| 184–231 | (exon 3) | ||
| 402–449 | (exon 4) | ||
| IL-12 | M86771 | 146–194 | (exon 1) |
| 595–642 | (exon 2) | ||
| M86672 | 190–238 | (exon 1) | |
| 706–753 | (exon 2) | ||
| IFN-γ | M29315 | 298–345 | (exon 1) |
| M29316 | 80–125 | (exon 2) | |
| M29317 | 303–350 | (exon 3) | |
| 180–227 | (exon 4) | ||
| TGF-β | X02812 | 1363–1410 | (exon 1) |
| 1457–1504 | (exon 2) | ||
| 1766–1813 | (exon 3) | ||
| 1953–2000 | (exon 4) |
Fig. 1.
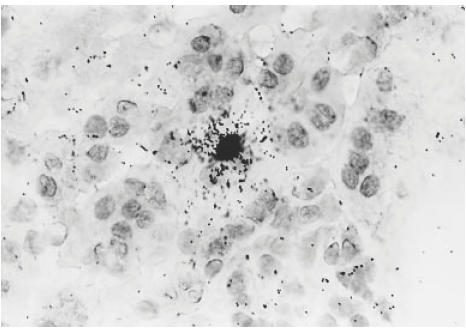
The juxtamedullary portion of the renal cortex of a mouse kidney. In situ hybridization of transforming growth factor-beta (TGF-β) mRNA overlying a tubular epithelial expressing cell. Mag. ×400.
EIA
Cytokine levels were determined in the supernatants of homogenates. Human TGF-β EIA kits were obtained from R&D Systems (Abingdon, UK). Although human TGF-β1 was used as a control, the assay kit has previously been shown also to detect murine TGF-β1 with equivalent accuracy [17]. The limit of detection was defined as 31·2 pg/ml.
Incubation of splenocytes with rIL-6
Splenocytes (105 cells/ml) from IL-6 knock-out and wild-type mice infected with E. coli and killed after 48 h, were incubated for 24 h at 37°C with 5, 10 and 15 ng/ml murine recombinant IL-6 (rIL-6) (R&D Systems). Supernatants were analysed for TGF-β by EIA.
Statistical analysis
The Mann–Whitney U-test was used to evaluate the statistical significance between infected and control groups.
Results
Increased mortality to E. coli in IL-6-deficient mice
Survival of infected IL-6-deficient mice and wild-type mice was measured over time. IL-6-deficient mice were more susceptible to death from intravesical challenge with 50 μl E. coli 109 CFU/ml: 14/78 (18%) infected mice died compared with 4/65 (6%) wild-type mice (P < 0·05). Mortality was rapid, with all deaths occurring within 24 h of infection.
Extensive bacterial growth in the kidneys of IL-6-deficient mice
After 48 h of infection, growth of E. coli was found in the kidneys of 4/5 knock out and 3/4 wild-type mice, indicating a good attack rate in both groups. After 6 days on the other hand, no bacterial growth was detected in the kidneys from wild-type animals, while four of five of the knock-out mice still had such bacterial growth (P < 0·05). One of the four IL-6-deficient mice, but none of the wild-type mice, also had bacterial growth 8 weeks post-infection.
Histopathological kidney changes similar but more severe in IL-6-deficient than in wild-type animals
The inflammatory changes observed were most often localized in the pelvic mucosa. Pyelitic changes in several kidneys were however, seen together with inflammation which was confined to the fornices with spreading into the adjacent parenchyma. More severe pyelonephritic changes involving the papilla and cortex were seen in a few kidneys. Inflammatory changes were observed after 48 h in 9/16 kidneys from IL-6-deficient mice and in 7/15 wild types with a mean inflammatory score of 1·13 and 0·47, respectively. After 6 days, inflammatory changes were observed in 4/5 kidneys of the knock-out mice (mean score 2·0) and in 2/4 of the wild-type animals (mean score 0·5). After 8 weeks, changes were observed in 2/4 kidneys from the knock-out mice (mean score 1·25) and in 0/4 of kidneys from the wild-type animals (mean score 0). In conclusion, the inflammatory changes were more frequent and, when present, more wide-spread in the IL-6-deficient mice compared with the wild-type animals (Fig. 2a,b,c).
Fig. 2.
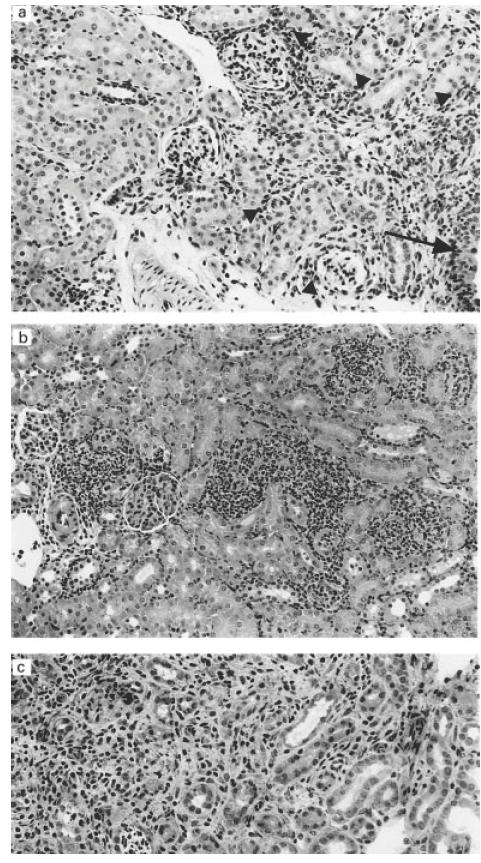
Kidney changes after infection with Escherichia coli. (a) Inflammation (arrowheads) confined to the fornices. The arrow marks the small part of the pelvic urothelium. (b) Streaks of acute inflammation with granulocytes in cortex found in an IL-6-deficient mouse 48 h post-infection. (c) Cortical area with inflammation, tubular atrophy and interstitial fibrosis in an IL-6-deficient mouse 8 weeks post-infection. Haematoxylin and eosin staining. Mag. ×250 (a,b), ×330 (c).
Increased TGF-β in kidney tissue from wild-type compared with IL-6-deficient mice
TGF-β mRNA expression and protein production were evaluated in IL-6-deficient and wild-type mice. Forty-eight hours after infection with E. coli a significant increase in TGF-β mRNA expression was observed in kidneys from wild-type (n = 8) compared with IL-6-deficient mice (n = 8) (P < 0·0008) (Fig. 3). At the same time point, an increase in TGF-β protein levels was also seen in the supernatant of homogenized kidneys in E. coli-infected wild-type (n = 4) compared with IL-6-deficient mice (n = 5) (P < 0·03). Similarly, when mice were injected with NaCl 0·9% (w/v), TGF-β mRNA expression was higher in the wild-type than in knock-out mice (P < 0·02). We hereby demonstrate the impact of IL-6 on the expression and protein production of TGF-β. Interestingly, TGF-β mRNA levels remained stationary throughout the 8-week study in the E. coli-infected IL-6-deficient mice, but were decreased, compared with the 48 h peak, after both 6 days and 8 weeks in the wild-type counterparts (P < 0·001, respectively) (Fig. 3). This increase in TGF-β mRNA levels was more pronounced at 48 h in E. coli-infected, compared with NaCl-injected, mice in both the wild-type (P < 0·01) and IL-6-deficient strains (P < 0·01), emphasizing the pronounced effect of E. coli on TGF-β expression. When mice were injected with NaCl 0·9% (w/v) and exposed to a temporary obstruction, TGF-β mRNA levels also increased in wild-type (n = 4) as well as in the IL-6 knock-out animals (n = 4) compared with untouched controls (P = 0·01, respectively) (Fig. 4), showing the impact of obstruction per se.
Fig. 3.
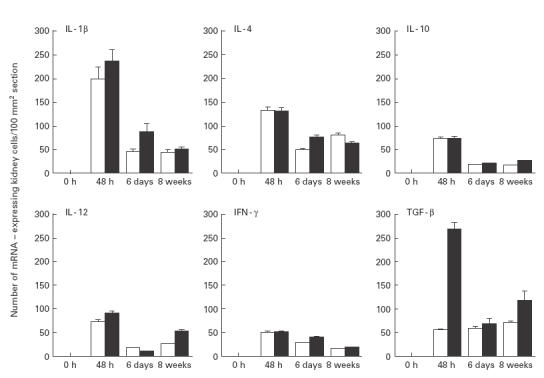
Cytokine mRNA expression in kidney sections. □, IL-6-deficient, ▪, wild-type animals infected with Escherichia coli and subjected to urethral obstruction for 6 h. Mean and s.e.m. are given.
Fig. 4.
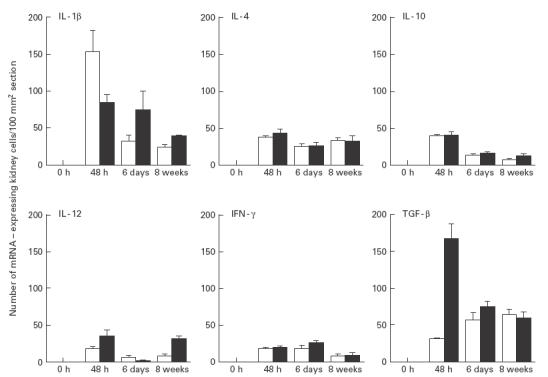
Cytokine mRNA expression in kidney sections. □, IL-6-deficient, ▪, wild-type animals injected with NaCl (0·9% w/v) and subjected to urethral obstruction for 6 h. Mean and s.e.m. are given.
rIL-6 restored TGF-β levels in splenocytes of IL-6-deficient mice
Splenocytes from E. coli-infected mutant and wild-type mice were incubated with varying doses of murine rIL-6. A significant increase in TGF-β levels was observed with the lowest dose used (5 ng/ml), and a further slight increase was obtained with 10 and 15 ng/ml in splenocytes from the IL-6-deficient mice (P < 0·05), while no such increase was seen with any of the given doses in splenocytes from the wild-type mice. At the lowest rIL-6 dose, TGF-β levels from IL-6-deficient splenocytes reached those of wild-type splenocytes and no significant differences were observed (Fig. 5).
Fig. 5.
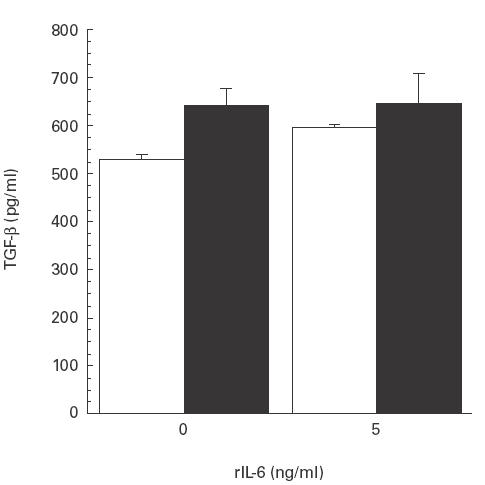
Transforming growth factor-beta (TGF-β) production analysed by enzyme immunosorbent assay (EIA) in supernatants from splenocytes incubated with murine rIL-6. A significant increase was seen with the lowest dose (5 ng/ml) of rIL-6, while no such increase was observed for splenocytes from wild-type mice. □, Splenocytes from IL-6-deficient mice; ▪, wild-type animals. Mean and s.e.m. are given.
Peak cytokine mRNA expression 48 h after E. coli infection in IL-6-deficient and wild-type animals
After 48 h, E. coli-infected and obstructed IL-6-deficient and wild-type mice displayed peak mRNA expression of IL-1β, IL-4, IL-10, IL-12 and IFN-γ in the kidneys. A significant decrease in expression of the studied cytokines was observed in both groups at 6 days or 8 weeks (P < 0·05, respectively) compared with the peak level (Fig. 3). Similarly, in mice injected with NaCl 0·9% (w/v) and temporarily obstructed, the peak expression of these cytokines also occurred after 48 h in both the IL-6-deficient and wild-type mice. A decrease followed at 6 days and 8 weeks (P < 0·05, respectively) (Fig. 4). At 48 h, mRNA expression of all the studied cytokines was lower in the NaCl 0·9% (w/v)-injected IL-6-deficient and wild-type mice compared with the E. coli-infected mice (P < 0·05, respectively). IL-6 was detected in the wild-type mice, with a kinetic pattern similar to the other cytokines. In none of the mutant mice at any time point however, was IL-6 detected.
Discussion
In the present study, we demonstrate that IL-6-deficient mice with acute pyelonephritis were less efficient at resolving E. coli infections compared with their wild-type counterparts and that this incapacity resulted in an increased mortality rate. The IL-6-deficient mice developed greatly increased bacterial loads in their kidneys, with more severe and generalized histopathological changes. TGF-β gene expression and protein secretion were increased in the E. coli-infected wild-type mice, compared with the IL-6-deficient mice. Interestingly, NaCl injection combined with a temporary obstruction of the urethra produced an increase in TGF-β mRNA with similar kinetics but at a lower level.
During acute pyelonephritis, bacteria proliferate and spread throughout the kidneys [11,18], although not all nephrons are affected to the same extent. Likewise, IL-6 production varies with the intensity of bacterial proliferation [11]. The infection induces a neutrophil migration across the uroepithelial cell layers directed by chemokines such as IL-8. Consequently, increased urinary IL-8 levels and neutrophil responses accompany acute pyelonephritis [7,8]. It is well recognized that neutrophils are important in the anti-microbial host defence of systemic infections [19,20]. Interestingly, IL-6-deficient mice are unable to induce neutrophilia in response to either Gram-negative [21] or Gram-positive [22] infections, although there is no difference in the overall leucocyte count between IL-6-deficient and wild-type mice [21]. This initial lack of an adequate neutrophil response is critical and may therefore be an important general aspect of innate immunity. Mice depleted of neutrophils, but with an intact IL-6 gene, were highly susceptible to E. coli infections, displaying a 1000-fold increase of bacteria compared with controls [19], proving the importance of neutrophils during infections.
In the present study, the increased susceptibility to E. coli infection in IL-6 knock-out mice was striking and resulted in a higher mortality rate within the first day. We also found a lack of renal bacterial clearance in the IL-6 knock-out mice after 6 days. Similarly, previous studies showed that IL-6-deficient mice were unable to clear E. coli [21] and Listeria infection from the liver and spleen after i.p. injection [23]. It is known that IL-6 may be involved in the regulation of endogenous anti-microbial peptides [24] of possible importance for bacterial clearance in pyelonephritis. Since the expression of endogenous anti-microbial peptides is under NF-κB regulation [25], tumour necrosis factor-alpha (TNF-α) and IL-1 and therefore also indirectly IL-6 may regulate anti-microbial peptide production via this route [26]. The protective role of IL-6 has been further elucidated by showing that when rIL-6 is given before i.v. challenge of Listeria the infection is suppressed [27] and mortality reverted [22].
Urethral obstructive malformation is the most important cause of renal failure in children. The obstruction may promote vesicourethral reflux into the renal pelvis and tubular system of the kidneys. In association with urinary tract infections renal damage often occurs [28]. In the present study we demonstrate a significant increase in TGF-β levels due to obstruction per se and further up-regulation when mice were infected with E. coli. TGF-β has a central role in the regulation of the glomerular matrix components fibronectin, laminin and type IV collagen and is therefore of great importance for the development of renal damage [29]. Major emphasis has been placed on the possibility of reducing its production, thereby decreasing the matrix metalloproteinases and also renal fibrosis. We demonstrate here that TGF-β is markedly lower in IL-6-deficient mice. This reduction was reversed by supplementation with recombinant IL-6, demonstrating an involvement of IL-6 in the regulation of TGF-β expression.
IL-6 has been postulated to play a negative feedback role: down-regulation of TNF-α and IL-1β was seen at the transcriptional level in serum upon challenging mice with lipopolysaccharide (LPS) [30,31]. In the present study no such decrease in IL-1β expression was shown, which may be explained by the different mechanisms by which LPS and whole live bacteria act on the localized tissue, as well as by cell and tissue specificity. Bacterial LPS has systemic rather than local effects and bypasses the crucial immune response by triggering inflammatory mediators from a variety of cell types and may therefore be less dependent on IL-6 [23]. Although IL-6 is irrelevant for survival during LPS-induced septic shock [24], it plays a protective role in the immune response against E. coli infection. Similarly, the liver acute-phase response assessed in the serum of IL-6-deficient mice treated with LPS was only slightly reduced compared with controls. By contrast, in animals with Listeria infections a moderate [23], and after tissue damage a striking [32], reduction was observed.
It is tempting to speculate that defective leucocyte recruitment and possibly decreased anti-microbial peptide activity contributes to the impaired innate immunity and reduced resistance to microbial agents seen in IL-6-deficient mice. IL-6 stimulates TGF-β expression, which may be detrimental to the kidneys in young children. The increase in TGF-β expression after non-infected obstruction is therefore interesting. We here illustrate an early beneficial effect of IL-6 coupled with the harmful effects and risk of developing renal damage induced by TGF-β. Our findings show the complexity of the clinical outcome invoked by the cytokine response to bacterial infection and urethral obstruction and underline the difficulties linked with cytokine treatment.
Acknowledgments
Supported by Funds from the Karolinska Institute, Magn. Bergvalls Foundation and Stiftelsen Frimurare Barnhuset. A.K. had an educational grant from Merck Sharp & Dohme, Sweden.
References
- 1.Van Snick J. Interleukin-6: an overview. Ann Rev Immunol. 1990;8:253–78. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 2.Okada M, Kitahara M, Kishimoto S, Matsuda T, Hirano T, Kishimoto T. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol. 1988;141:1543–9. [PubMed] [Google Scholar]
- 3.Ruppert J, Peters JH. IL-6 and IL-1 enhance the accesory activity of human blood monocytes during differentiation to macrophages. J Immunol. 1991;146:144–9. [PubMed] [Google Scholar]
- 4.Barton BE, Jackson JV. Protective role of interelukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993;61:1496–9. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–7. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 6.Stahl N, Yancopoulos GD. The alphas, betas, and kinases of cytokine receptor complexes. Cell. 1993;74:587–90. doi: 10.1016/0092-8674(93)90506-l. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson SH, Hylander B, Wretlind B, Brauner A. Interleukin-6 and interleukin-8 in serum and urine in patients with acute pyelonephritis in relation to bacterial virulence associated traits and renal function. Nephron. 1994;67:172–9. doi: 10.1159/000187923. [DOI] [PubMed] [Google Scholar]
- 8.Tullus K, Wang J-A, Lu Y, Burman LG, Brauner A. Interleukin-1α and interleukin-6 in the urine, kidney and bladder of mice inoculated with E. coli. Pediatr Nephrol. 1996;10:453–7. doi: 10.1007/s004670050138. [DOI] [PubMed] [Google Scholar]
- 9.Khalil A, Brauner A, Bakhiet M, Burman LG, Jaremko G, Wretlind B, Tullus K. Cytokine expression during experimental Escherichia coli pyelonephritis in mice. J Urol. 1997;158:1576–80. [PubMed] [Google Scholar]
- 10.Rugo HS, O'hanley P, Bishop AG, Pearce MK, Abrams JS, Howard M, O'garra A. Local cytokine production in a murine model of Escherichia coli pyelonephritis. J Clin Invest. 1992;89:1032–9. doi: 10.1172/JCI115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaboré AF, Simard M, Bergeron MG. Local production of inflammatory mediators in an experimental model of acute obstructive pyelonephritis. J Infect Dis. 1999;179:1162–72. doi: 10.1086/314700. [DOI] [PubMed] [Google Scholar]
- 12.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X-F, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–20. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karkar AM, Smith J, Tam FW, Pusey CD, Rees AJ. Abrogation of glomerular injury in nephrotoxic nephritis by continuous infusion of interleukin-6. Kidney Int. 1997;52:1313–20. doi: 10.1038/ki.1997.456. [DOI] [PubMed] [Google Scholar]
- 14.Tullus K, Fituri O, Linné T, Escobar-Billing R, Karlsson A, Burman LG, Wretlind B, Brauner A. Urine interleukin-6 and interleukin-8 in children with acute pyelonephritis, in relation to DMSA scintigraphy in acute phase and at 1-year follow-up. Pediatr Radiol. 1994;24:513–5. doi: 10.1007/BF02015016. [DOI] [PubMed] [Google Scholar]
- 15.Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13:1189–96. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson T, Bakhiet M, Höjeberg B, et al. CD8 is critically involved in lymphocyte activation by a T. Brucei brucei-released molecule. Cell. 1993;72:715–27. doi: 10.1016/0092-8674(93)90400-k. [DOI] [PubMed] [Google Scholar]
- 17.Maltman J, Pragnell IB, Graham GJ. Specificity and reciprocity in the interactions between TGF-β and macrophage inflammatory protein-1α. J Immunol. 1996;156:1566–71. [PubMed] [Google Scholar]
- 18.Serlachius E, Sundelin B, Eklöf A-C, Jahnke M, Laestadius Å, Aperia A. Pyelonephritis provokes growth retardation and apoptosis in infant rat renal cortex. Kidney Int. 1997;51:1855–62. doi: 10.1038/ki.1997.253. [DOI] [PubMed] [Google Scholar]
- 19.Hang L, Haraoka M, Agace W, Leffler H, Burdick M, Strieter R, Svanborg C. Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J Immunol. 1999;162:3037–44. [PubMed] [Google Scholar]
- 20.Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–6. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalrymple SA, Slattery R, Aud DM, Krishna M, Lucian LA, Murray R. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect Immun. 1996;64:3231–5. doi: 10.1128/iai.64.8.3231-3235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalrymple SA, Lucian LA, Slattery R, McNeil T, Aud DM, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–8. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–41. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 24.Frohm-Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Ståhle-Bäckdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–6. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmaco M, Boman A, Mangoni ML, Migogna G, Miele R, Barra D, Boman HG. Effect of glucocorticoids on the synthesis of antimicrobial peptides in amphibian skin. FEBS Letters. 1997;416:273–5. doi: 10.1016/s0014-5793(97)01216-7. [DOI] [PubMed] [Google Scholar]
- 26.Baldwin As., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Ann Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Simpson RJ, Cheers C. Recombinant interleukin-6 protects mice against experimental bacterial infection. Infect Immun. 1992;60:4402–6. doi: 10.1128/iai.60.10.4402-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin N Am. 1997;11:609–22. doi: 10.1016/s0891-5520(05)70376-7. [DOI] [PubMed] [Google Scholar]
- 29.Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987;105:1039–45. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aderka D, Le J, Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989;143:3517–23. [PubMed] [Google Scholar]
- 31.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlation and interactions in the production of interleukin-6 (IL-6), IL-1 and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–47. [PubMed] [Google Scholar]
- 32.Fattori E, della Rocca C, Costa P, Giorgio M, Dente B, Pozzi L, Ciliberto G. Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood. 1994;83:2570–9. [PubMed] [Google Scholar]


