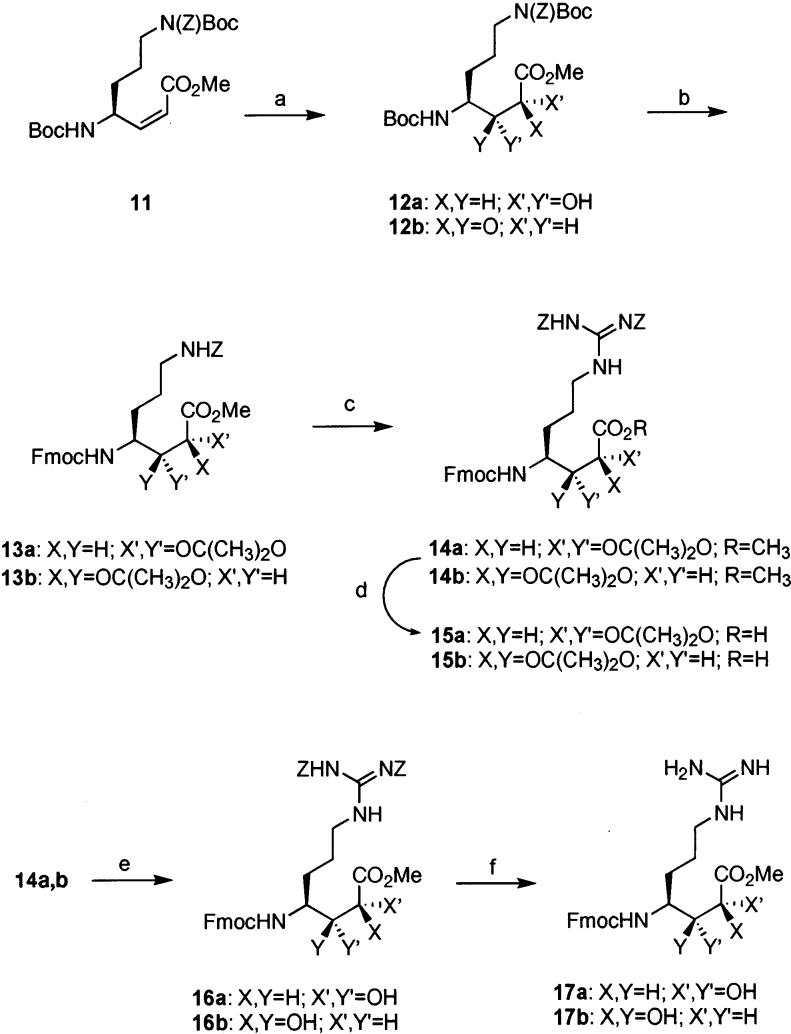
Scheme 3a.
a Reagents and conditions: (a) OsO4, NMO, MeSO2NH2, acetone–tBuOH–H2O (12a, 49%; 12b, 49%); (b) (i) 33% TFA–CH2Cl2, (ii) Fmoc–OSu, NEt3, CH2Cl2 (82%; 73%, two steps), (iii) CSA, (CH3)2C(OCH3)2, DMF, 35 °C (13a, 78%; 13b, 89%); (c) (i) H2, Pd/C, 5% AcOH–MeOH, (ii) N,N′-di-Z-N″-trifylguanidine, NEt3, CHCl3 (14a, 66%; 14b, 72%, two steps); (d) LiOH, THF, 0 °C (15a, 90%; 15b, 90%); (e) Dowex resin, 90% MeOH–H2O (16a and 16b in quantitative yield); (f) H2, Pd/C, 2.5% AcOH–MeOH (17a, 78%; 17b, 70%).