Figure 5.
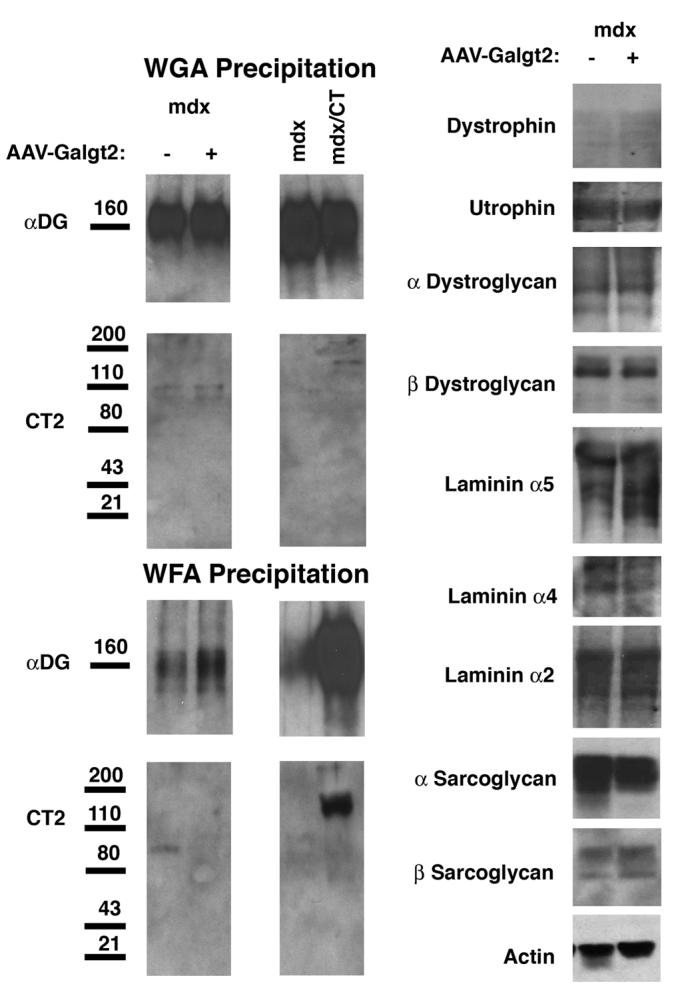
Postnatal overexpression of Galgt2 does not increase CT glycosylation of α dystroglycan or expression of utrophin, synaptic laminins, or dystrophin-associated glycoproteins in mdx muscle.
Left, 150μg of NP-40 detergent protein lysate was precipitated with a GalNAc-binding lectin that can identify the CT carbohydrate (WFA agarose) or with a control lectin known to precipitate α dystroglycan (WGA agarose). Proteins were eluted with GalNAc (for WFA) or GlcNAc (for WGA) and analyzed by Western blot for α dystroglycan or CT carbohydrate (CT2). Postnatal overexpression of Galgt2 using AAV caused a slight increase in α dystroglycan precipitated with WFA, but this protein was not glycosylated with the CT carbohydrate. By contrast, WFA precipitated CT-glycosylated α dystroglycan from Galgt2 transgenic mdx muscle (mdx/CT). WGA precipitation showed equal amounts of α dystroglycan in each pair of samples. Right, comparison of whole cell muscle lysates showed postnatal overexpression of Galgt2 did not increase expression of utrophin, α dystroglycan, β dystroglycan, laminin α2, laminin α4, laminin α5, α sarcoglycan, or β sarcoglycan protein. Blots were stripped and reprobed for actin as a control for protein loading and transfer.
