FIGURE 4. Morphine tolerance under a separate morphine regimen (Kest et al., 2002) following a within-subjects design and cumulative dosing or a between-subjects design and a single challenge dose.
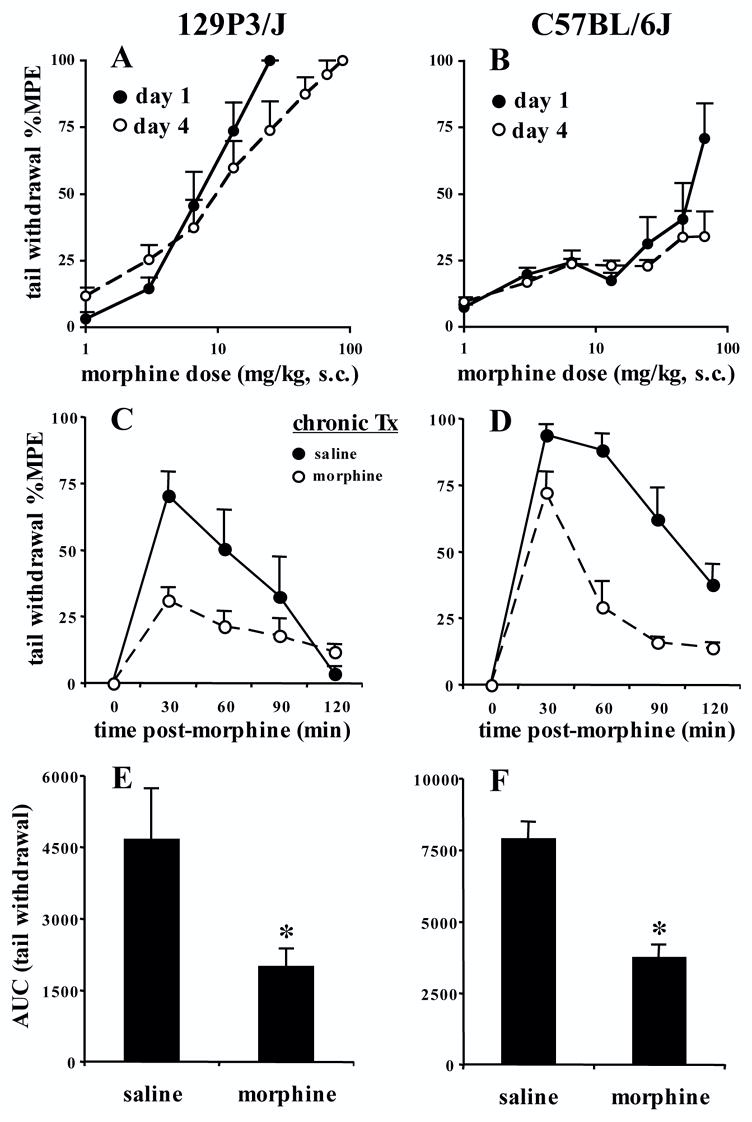
Mice (N=8) were assayed for baseline latencies on day 1, and immediately administered morphine (s.c.). Thirty min later, immediately following post-injection latency assessment mice, were administered a subsequent dose of morphine. This was repeated every 30 min. Doses of 1, 2, 3.6, 6.5, 11.7, and 21.0 mg/kg were administered. In the case of extra injections, 21.0 mg/kg was administered (Kest et al. 2002). All mice received the same number of injections and doses of morphine on day 1, although if they reached cut-off, they were not subjected to further pain testing (Kest et al. 2002). The x-axis represents the cumulative morphine dose. A and B). No tolerance developed in 129P3/J mice in the tail withdrawal assay as indicated by a lack of shift in the dose-response curve. Comparable ED50 values were obtained between days 1 and 4 (see Results). ED50 estimates for C57BL/6J mice were not possible, due to the lack of comparable maximum possible effects that could be reached, even with subsequent dosing on day 4 (data not shown). C and D). Tolerance developed in 129P3/J and C57BL/6J mice in the tail withdrawal assay following a between-subjects design, the regimen of Kest et al. (2002), and a single challenge dose of morphine on test day (129P3/J = 7.5 mg/kg, s.c.; C57BL/6J = 25 mg/kg, s.c.) . E and F). Tolerance as indicated by AUC in 129P3/J and C57BL/6J mice. Data are presented as the mean ± S.E.M. A p value of 0.05 was considered significant ("*").
