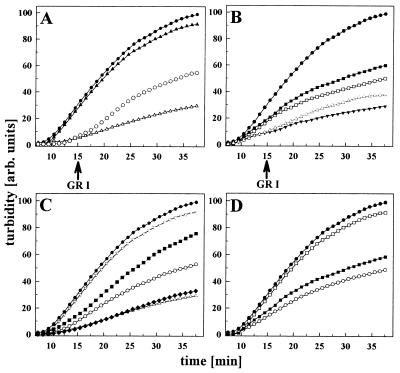
Figure 3.
Substrate specificity of the Hsp90 chaperone sites. The effects of Hsp90 and fragments thereof (45 μM each) on insulin aggregation (45 μM) were monitored in the presence of various peptides. (A) Influence of GR1 peptide (45 μM) on insulin binding to N210. Aggregation of insulin in the absence (•) and in the presence of N210 (▵), N210 + GR1 (▴), and addition of GR1 (indicated by the arrow) to a preformed N210 insulin complex (○). (B) Influence of GR1 peptide on insulin binding to 262C. Aggregation of insulin in the absence of Hsp90 (•), in the presence of 262C (▾), 262C + 45 μM GR1 (□), and 262C + 225 μM GR1 (▪) and addition of GR1 to a preformed 262C⋅insulin complex (▵). (C) Influence of various peptides on insulin binding to N210. Aggregation of insulin in the absence of Hsp90 (•) and in the presence of N210 (▵), N210 + GR1 (▿), N210 + VSV8 (▪), N210 + HD 131 (○), and N210 + HD25 (⧫). (D) Comparison of the influence of peptide on the chaperone activity of wild-type Hsp90 and fragments thereof. Aggregation of insulin in the absence of Hsp90 (•) and in the presence of Hsp90 + GR1 (▪), 262C + GR1 (○), and N210 + GR1 (□). Concentrations of the fragments were 45 μM, and concentration of wild-type Hsp90 was 18 μM (based on the respective monomers). GR1 was added at a 1:1 ratio to insulin.