Abstract
The immunological correlates of highly active antiretroviral therapy (HAART)-induced suppression of human immunodeficiency virus type 1 (HIV-1) replication have been investigated.
20 HIV-1-infected patients with mean CD4+ T cell count of 298/µl, plasma viral load of 4·7 log10 copies/ml and naive for protease inhibitors (PI) were studied during12 months of HAART. An increased number of both CD4+ and CD8+ naive T cells and a normalization of the frequency of CCR5- and CXCR4-expressing CD4+ T cells were readily observed after starting therapy. Single cell analysis of cytokine production after 12 months of HAART showed an increased number of interleukin (IL)-2-, but not IL-4- and (IFN)-γ-, producing T cells and a decreased percentage of CD8+ IFN-γ + cells. A correlation between the frequency of IFN-γ-producing T cells and that of memory, CCR5+ and CD95+ T cells was demonstrated in both CD4+ and CD8+ subsets. The diversity of T cell receptor (TCR) variable beta (BV) chain repertoire significantly increased after 12 months of HAART within the CD4+ but not the CD8+ T cell subset. However, the level of perturbation of the third complementarity-determining region (CDR3), was not significantly modified by effective therapy. The number of anti-HIV Gag and Pol cytotoxic T lymphocytes precursors (CTLp) decreased during HAART and highly correlated with the CD8 IFN-γ response. Ameliorated clinical conditions were observed in all patients in absence of any opportunistic infections during all the study period. These observations indicate that a better restoration of immunity may be obtained in patients starting HAART at less advanced stages of the disease.
Keywords: HIV, HAART, cytokine, CTL, TCR
INTRODUCTION
Treatment with highly active antiretroviral therapy (HAART), combining HIV protease inhibitors (PI) and reverse transcriptase inhibitors (RTI), can suppress HIV replication both in the circulation and in lymphoid tissues and improve both CD4+ T cell count and function [1]. However, in spite of intense investigation, the mechanisms underlying HAART-induced immune reconstitution remain to be fully characterized. Initial studies performed on HIV-1 infected patients with advanced disease suggested that HAART-induced T cell repopulation was mainly due to an early recirculation of memory cells from lymphoid tissues to blood, accompanied by a slow production of newly generated naive T cells [2,3]. More recently, a correlation between the size of thymic tissue and the magnitude of naive T cell recovery after HAART has been demonstrated, suggesting a critical role for the thymus in lymphocyte restoration [4]. This finding is of remarkable importance considering that an effective immune reconstitution is possible only if new naive cells, with a wider repertoire, are generated. Previous studies, designed to assess the impact of HAART on the immune system, have yielded the conclusion that, although with some notable exceptions [5], HIV-induced impairment of immune functions is relatively stable [6,7]. However, it should be noted that these studies were performed on patients with advanced HIV disease.
Since immunological factors influencing the decision to start therapy are still debated it would be of great clinical importance to establish whether a more complete immune reconstitution might be achieved in patients starting HAART at less advanced stages of the disease.
In order to answer this question, some of the most notable HIV-1 induced immune abnormalities have been investigated before and during 12 months of HAART in HIV-1 infected patients with moderate immunodeficiency and naive for PI.
Among the immune defects contributing to the AIDS immunopathogenesis the dysregulation of the cytokine network, has been proposed to play a key role [8,9]. The decreased diversity of the CD4+ T cell receptor (TCR) repertoire, arizing from HIV-1-induced deletion of CD4+ T cell clones, has been also invoked as responsible for loss of antigen specific responsiveness [10]. In addition, anti-HIV cytotoxic activity, that plays a fundamental role in limiting virus spread during the early phases of infection, generally decreases with time, in association with disease progression [11]. Finally, chemokine receptors, in addition to regulate the HIV entry into the target cells, could also contribute to AIDS immunopathogenesis [12]. All these immunological correlates of HIV infection, previously investigated in individual reports [5,13–15], have been here perspectively studied in order to provide a more comprehensive evaluation of HAART-induced immune reconstitution.
MATERIALS AND METHODS
Study population
Twenty HIV-1-infected patients were selected randomly among a population belonging to a larger clinical trial whose inclusion criteria were: CD4+ T cell count >100 cell/µl <500 cell/µl, HIV-RNA plasma viraemia >10·000 copies/ml and no previous treatment with protease inhibitors [16]. Fifteen patients were males and 5 were females. The median age at entry was 38 years (range 29–56 years). According to risk factors, the patients were stratified as follows: 9 heterosexual, 6 homosexual, and 5 ex-intravenous drug users. Seven out of 20 subjects were nucleoside reverse transcriptase inhibitors (NRTI)-experienced (mean previous therapy with NRTI was 1·4 years). No concomitant active opportunistic infections were present at enrolment and during the follow-up. Karnofsky's score, clinical signs and symptoms of HIV-related and AIDS-defining events were monitored monthly. Blood samples were taken on entry and after 3, 6 and 12 months. Detailed clinical and laboratory characteristics of patients under investigation are shown in Table 1. All subjects gave informed written consent according to the Ethical Committee of the University of Rome ‘La Sapienza’, Italy.
Table 1.
Baseline clinical and laboratory characteristics
| Patient no. | Age (years) | Sex | Risk factors | Disease stage* | CD4 (cells/µl) | Viral load (log copies/ml) | Antiretroviral treatment |
|---|---|---|---|---|---|---|---|
| 1 | 56 | M | Homosexual | A2 | 287 | 4·27 | AZT + 3TC + IDV |
| 2 | 31 | F | Heterosexual | A1 | 511 | 4·40 | AZT + 3TC + IDV |
| 3 | 36 | M | Intravenous drug user | B3 | 197 | 4·66 | 3TC + D4T + IDV |
| 4 | 30 | F | Heterosexual | A2 | 349 | 4·75 | AZT + 3TC + RTV |
| 5 | 52 | M | Homosexual | B2 | 228 | 4·70 | 3TC + D4T + IDV |
| 6 | 35 | M | Intravenous drug user | B3 | 181 | 4·85 | 3TC + D4T + IDV |
| 7 | 38 | M | Intravenous drug user | B2 | 263 | 5·85 | 3TC + D4T + IDV |
| 8 | 35 | M | Homosexual | B2 | 474 | 4·89 | AZT + 3TC + IDV |
| 9 | 29 | F | Heterosexual | A2 | 475 | 3·90 | 3TC + D4T + IDV |
| 10 | 37 | F | Intravenous drug user | B2 | 282 | 4·34 | 3TC + D4T + RTV |
| 11 | 43 | M | Heterosexual | B2 | 434 | 4·23 | 3TC + D4T + IDV |
| 12 | 33 | F | Heterosexual | A2 | 334 | 4·04 | AZT + 3TC + IDV |
| 13 | 36 | M | Intravenous drug user | B2 | 252 | 4·85 | AZT + 3TC + IDV |
| 14 | 37 | M | Heterosexual | A3 | 138 | 4·66 | 3TC + D4T + IDV |
| 15 | 35 | M | Homosexual | A2 | 343 | 4·04 | AZT + 3TC + IDV |
| 16 | 35 | M | Homosexual | A2 | 228 | 4·56 | 3TC + D4T + RTV |
| 17 | 39 | M | Heterosexual | A2 | 286 | 4·92 | 3TC + D4T + IDV |
| 18 | 36 | M | Heterosexual | B3 | 172 | 5·71 | AZT + 3TC + IDV |
| 19 | 31 | M | Heterosexual | B3 | 141 | 5·53 | 3TC + D4T + IDV |
| 20 | 56 | M | Homosexual | C2 | 334 | 5·05 | AZT + 3TC + IDV |
According the 1993 revised AIDS definition [47]
Flow–cytometric analysis of CD4+ and CD8+ T cell subsets
All the immunological parameters reported in the following paragraphs were performed at enrolment (t0) and after 3, 6 and 12 months of therapy (t3, t6 and t12, respectively). Whole-blood phenotype analysis consisted of lysing 500 µl blood with 10 ml of Ortho-mune Lysing Reagent (Ortho Diagnostic Systems Inc., Raritan, NJ) at room temperature (RT), washed and labelled with a cocktail of four monoclonal antibodies (mAbs) for 30 min at 4°C. The peripheral redistribution of naive and memory T cells has been investigated by evaluating the differential expression of CD45RA and CD62L molecules on both CD4+ and CD8+ T cells. In fact, evaluation of CD45RA expression is not capable of fully discriminating naïve from memory T cells. In particular, it has been shown that memory T cells, expressing CD45R0, that fail to re-encounter the specific antigen may revert to a CD45RA+ phenotype [17]. However, the expression of CD62L remains downregulated in such ‘revertants’, thus allowing a more detailed determination of naive T cells [18]. Anti-CD4-allophycocyanin (APC), anti-CD8 peridinin chlorophyll protein (PerCP), anti-CD45RA-fluorescein isothiocyanate (FITC) were purchased from Becton Dickinson (San Jose, CA); anti-CD62L-PE, anti-CCR5-PE (clone 2D7) and anti-CXCR4-PE (clone 12G5) were purchased from Pharmingen (San Diego, CA); anti-Fas-FITC was obtained from MBL (Medical & Biological Laboratories Co., Ltd, Japan). After staining cells were washed once in phosphate-buffered saline (PBS) containing 2% foetal bovine serum (FBS) and analysed on a FACSCalibur cytofluorometer (Becton Dickinson, Mountain View, USA) using the Cell Quest software. To determine marker expression on CD4 and CD8 cells total lymphocytes were first identified and gated by forward and side scatter. The cells were then additionally gated for CD4 or CD8 expression. 10000 gated events were collected for each sample. Appropriate isotypic negative controls were run in parallel.
Single cell analysis of cytokine production
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood of HIV-infected individuals or normal donors by Ficoll-Isopaque (Lymphoprep-Nycomed, Oslo, Norway) gradient centrifugation, counted and cultured at 1 × 106 cells/ml in RPMI-1640 medium (Gibco Laboratories, Grand Island, NY) supplemented with 10% FBS (Sigma), 2 mm glutamine and antibiotics in a CO2 incubator at 37°C. Analysis of cytokine production at single cell level was performed as previously described [19]. The following cytokine-specific mAbs were used: FITC labelled antihIFN-γ (IgG2b), FITC labelled antihIL-2 (IgG1) and PE labelled antihIL-4 (IgG1). Isotype controls were labelled with FITC and PE. Surface phenotyping was performed with anti-CD4-APC and anti-CD8-PerCP. All the mAbs were purchased from Becton Dickinson. To determine the frequency of cytokine producing T cells, total lymphocytes were first gated by forward and side scatter and then additionally gated for CD4 or CD8 expression. 10·000 gated events were collected for each sample. Appropriate isotypic negative controls were run in parallel.
Analysis of TCRBV repertoire by cytofluorometry
Unfractionated T cells were incubated at 4°C for 30 min with the primary unlabeled anti-TCRBV Ab (Immunotech, Marseille, France) washed and incubated for additional 30 min in presence of goat anti mouse PE-labelled secondary Ab (Becton Dickinson, San Jose, CA). Cells were washed twice, incubated for 30 min with mouse serum, washed twice and finally incubated for 30 min with FITC-conjugated anti-CD4 or PercP-conjugated anti-CD8. Cells were then analysed for triple colour staining according to the isotype matched control.
Analysis of TCRBV repertoire by CDR3 spectratyping
CD4+ and CD8+ T cells from HIV-1 infected individuals and 5 HIV-negative blood donors were separated by using magnetic beads coated with anti-CD4 and anti-CD8 mAbs according to the manufacturer's protocols (Dynabeads, Dynal, Oslo, Norway). Total RNA was isolated using Trizol-LS Reagent (Life Technologies, Gibco-BRL, N.Y.) and Micro-carrier (Molecular Research Center, Cincinnati) and precipitated with isopropyl alcohol. The pelleted RNA was resuspended in diethyl-pyrocarbonate-treated water and the poly (A)+ portion of total RNA, was converted into cDNA using 2·5 µm oligo(dT) (Perkin Elmer, Norwalk, CT, USA) and 2·5 units MULV reverse transcriptase (Perkin Elmer). The sequences of 24 TCRBV subfamilies specific primers (BV 1, 2, 3, 4, 5·1, 5·3, 6·1, 6·2, 7, 8, 9, 11, 12, 13, 14, 15, 16, 17, 18, 20, 21, 22, 23, 24) and of a TCR constant β-chain (BC) primer used in this study were previously described by Maslanka et al. [20]. Amplications of target cDNA were performed as reported by Gorochov et al. [5]. 2 µl run-off products were mixed with 12 µl of deionized formamide and 0·5 µl Tamra 500 size standard (Perkin Elmer) and then electrophoresed on ABI Prism 310 (Perkin Elmer). PCR products were then analysed by using the Genescan software (Perkin Elmer). The percentage of each peak was then calculated and the perturbation of each CDR3 length evaluated by the difference between each sample's distribution and the control's distribution.
HIV-1 specific CTL responses
Recombinant Vaccinia virus (rVV) VVK1 which express HIV-1LAI gag and pol, was kindly provided by Dr O. Pontesilli (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, CLB). Vaccinia virus 186poly containing no insert was used as control. HIV-1 specific CTL precursors (CTLp) were expanded in vitro as described by [21]. Frequencies of CTLp specific for HIV Gag and Pol were determined using standard methods of limiting dilution analysis as previously described [15].
Determination of viral load
Plasma HIV RNA levels were determined by using the Roche Amplicor assay following the manufacturer's instructions. In patient with viral load <200 copies/ml (2·3 log), an ultrasensitive assay procedure was performed. This procedure, designed for use with the Amplicor HIV-1 Monitor Test, increases the analytical sensitivity of the test, thereby permitting detection of as few as 50 viral copies/ml (1·69 log) [22].
Statistical analysis
The data are presented as arithmetic mean ±SD. Baseline and follow-up data were compared using Wilcoxon-matched pairs test and for data of different groups Mann–Whitney test was used; P-values <0·05 were considered significant. Correlations were calculated by the Spearman's rank correlation test. With regard to TCRBV expression we define as expansion a value greater than the mean of the controls plus three standard deviations, which in the current study are referred to 15 normal blood donors. Differences in the number of expansions at each time-point were evaluated by the stratified data permutation analysis. To test the hypothesis whether the number of expansions are correlated with the number of CD4 cells or viral load a stratified permutation was performed, using both the Spearman's and Kendall's rank correlation tests.
RESULTS
Cytofluorometric analysis of CD4 and CD8 T cell subsets before and after HAART
All patients enrolled in the present study showed an optimal response to HAART in terms of viral load and CD4+ T cell count with the exception of patients no.17 and no.20 who experienced an immunological response in absence of a virological response. Mean viral load decreased from 4·71 ± 0·54 log at t0 to undetectable levels at t3 (limit of test detection 1·69 log) in 11 out of 20 patients and to 2·80 ± 1·11 in the remaining 9 patients. At t6 viral load became undetectable in 17 patients and was 3·3 ± 1·46 in the other 3 patients (Table 2). After 12 months of therapy viral load was undetectable in 16 out of 19 patients (patient no.20 dropped out for intolerable side-effects) and 3·59 ± 0·86 in the remaining 3 patients. This was seen in association with a significant rise in the absolute number of CD4+ and CD8+ T cells as well as in the percentage of CD4+ cells. As opposite, the percentage of CD8+ T cells was not significantly modified (Table 2).
Table 2.
Mean values (± SD) of CD3+CD4+, CD3+CD8+, naive (CD45RA+CD62L+), memory (CD45RA+CD62L−/CD45RA−CD62L+), CCR5+, CXCR4+ and CD95+ T cells and HIV-1 plasma viremia before (t0) and after 3 (t3), 6 (t6) and 12 (t12) months of HAART.
| CD4+ | CD8+ | |||||||
|---|---|---|---|---|---|---|---|---|
| t0 | t3 | t6 | t12 | t0 | t3 | t6 | t12 | |
| CD3+ (%) | 24 ± 10 | 26 ± 10 | 28 ± 9** | 28 ± 7* | 51 ± 9 | 48 ± 10* | 46 ± 9*** | 46 ± 8** |
| CD3+ (cells/µl) | 298 ± 111 | 445 ± 154*** | 537 ± 188*** | 497 ± 137*** | 642 ± 209*** | 906 ± 410*** | 935 ± 361** | 858 ± 381** |
| CD45RA+CD62L+ (%) | 52 ± 15 | 50 ± 16 | 50 ± 17 | 58 ± 15* | 38 ± 13 | 38 ± 16 | 44 ± 19** | 49 ± 20** |
| CD45RA+CD62L+ (cells/μl) | 148 ± 68 | 227 ± 125*** | 271 ± 150*** | 287 ± 104*** | 234 ± 75 | 312 ± 147** | 400 ± 184*** | 399 ± 170*** |
| CD45RA+CD62L−/CD45RA−CD62L+ (%) | 37 ± 10 | 39 ± 10 | 40 ± 14 | 34 ± 11 | 43 ± 10 | 43 ± 13 | 41 ± 14 | 39 ± 15 |
| CD45RA+CD62L−/CD45RA−CD62L+ (cells/µl) | 113 ± 56 | 170 ± 60*** | 213 ± 115*** | 165 ± 63** | 288 ± 138 | 410 ± 271* | 396 ± 252* | 360 ± 254 |
| CCR5+ (%) | 18 ± 7 | 14 ± 9** | 13 ± 7*** | 10 ± 6*** | 51 ± 14 | 38 ± 16*** | 40 ± 18** | 33 ± 12*** |
| CCR5+ (cells/μl) | 53 ± 29 | 59 ± 35 | 69 ± 44 | 48 ± 28 | 320 ± 109 | 353 ± 249*** | 378 ± 236*** | 280 ± 159*** |
| CXCR4+ (%) | 61 ± 10 | 55 ± 13* | 43 ± 9** | 53 ± 15* | 44 ± 17 | 37 ± 13 | 34 ± 13* | 34 ± 16* |
| CXCR4+ (cells/μl) | 189 ± 59 | 251 ± 112** | 256 ± 114** | 265 ± 109** | 265 ± 113 | 311 ± 163 | 323 ± 155 | 263 ± 139 |
| CD95+ (%) | 46 ± 15 | 47 ± 17 | 47 ± 17 | 46 ± 17 | 68 ± 16 | 66 ± 19 | 66 ± 17 | 66 ± 18 |
| CD95+ (cells/μl) | 139 ± 84 | 190 ± 84** | 248 ± 132*** | 227 ± 88*** | 446 ± 188 | 627 ± 396 | 625 ± 324* | 597 ± 353** |
| HIV1-RNA | 4·71 ± 0·54 | < 1·69 (11/20); | < 1·69 (17/20); | < 1·69 (16/19); | ||||
| (log10 copies/ml) | 2·80 ± 1·11(9/20) | 3·3 ± 1·46 (3/20) | 3·59 ± 0·86 (3/19) | |||||
Significance in comparison with baseline values:
P < 0·05
P < 0·01
P < 0·001
The HAART-induced changes in the peripheral distribution of naive (CD45RA+ CD62L+) and memory (CD45RA− CD62L+ and CD45RA+ CD62L−) cells, CCR5−, CXCR4− and CD95− expressing T cells are shown in Table 2. The absolute count of both naive and memory cells significantly increased in CD4 and CD8 subsets at t3, t6 and t12 with the exception of CD8 memory cells at t12. The increase in the percentage of naive cells reached statistical significance at t12 for CD4 and at both t6 and t12 for CD8 while no meaningful differences were observed in the percentage of both CD4+ and CD8+ memory cells. A significant decrease of the frequency of CCR5-expressing cells was induced by HAART in both CD4+ and CD8+ T cell subset, while the absolute numbers increased only up to t6 (Table 2). Similarly, the percentages, but not the absolute counts, of CXCR4-expressing cells decreased at all time points studied within both CD4 and CD8 subsets. Finally, a significant rise of CD95-expressing T cells was induced by HAART while the peripheral distribution of these cells remained unchanged (Table 2).
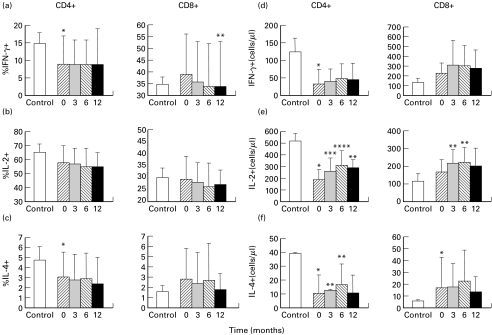
Single cell analysis of IFN-γ – producing T cell
The frequency of CD4+ and CD8+ IFN-γ producing T cells before and after HAART is shown in Fig. 1(a). A significant decrease in the percentage of CD4+ IFN-γ-expressing T cells was observed in HIV-infected patients as compared to controls (9 ± 8% versus 15 ± 3%, P = 0·03). As opposite, within the CD8+ subset, the peripheral distribution of IFN-γ-expressing cells was comparable to controls (39 ± 17% versus 35 ± 3%, P = 0·73). No changes were induced by HAART in the mean percentage of CD4+ IFN-γ-producing T cells (9 ± 7% at t3, 9 ± 7% at t6 and 9 ± 10 at t12). Within the CD8+ subset the percentage of IFN-γ-producing T cells progressively decreased: 36 ± 18% at t3, 34 ± 18% at t6 and 34 ± 19% at t12 (P = 0·02 for t12 versus t0). These cells progressively increased, as absolute numbers, after therapy, but not significantly (Fig. 1d, for CD4: from 33 ± 42 cells/µl at baseline to 40 ± 37 cells/µl at t3, P = 0·15, 49 ± 44 cells/µl at t6, P = 0·07 and 46 ± 47 cells/µl at t12, P = 0·33; for CD8: from 226 ± 107 cells/µl at baseline to 314 ± 255 cells/µl at t3, P = 0·17, 305 ± 207 cells/µl at t6, P = 0·14 and 279 ± 194 at t12 P = 0·34). Although the frequency of IFN-γ producing cells was generally decreased, in some patients the proportion of these cells increased during therapy (data not shown).
Fig. 1.
Mean percentage and absolute counts of IFN-γ (a,d), IL-2 (b,e) and IL-4 (c,f) producing cells within CD4+ and CD8+ T subsets at baseline (t0, ) and after 3 (t3, ▪), 6 (t6,
) and after 3 (t3, ▪), 6 (t6, ) and 12 (t12, ▪) months of HAART. Statistic significance between patients and HIV-negative (□) controls: *P < 0·05. Statistic significance of the follow-up values in comparison with baseline values: **P < 0·05, ***P < 0·01, ****P < 0·001.
) and 12 (t12, ▪) months of HAART. Statistic significance between patients and HIV-negative (□) controls: *P < 0·05. Statistic significance of the follow-up values in comparison with baseline values: **P < 0·05, ***P < 0·01, ****P < 0·001.
A significant correlation was demonstrated, in both CD4+ and CD8+ T cells, between the percentages of IFN-γ producing cells and memory (for CD4 r = 0·61, P < 0·0001 and for CD8 r = 0·40, P = 0·002), CCR5 + (for CD4 r = 0·52, P < 0·0001 and for CD8 r = 0·45, P = 0·0004) and CD95+ (for CD4 r = 0·76, P < 0·0001 and for CD8 r = 0·66, P < 0·0001) T cells.
Single-cell analysis of IL-2-producing T cells
Data regarding the distribution of IL-2-producing cells are shown in Fig. 1(b,e). The percentage of IL-2-expressing CD4+ and CD8+ T cells was similar in HIV-infected patients and in controls (for CD4: 58 ± 12% versus 65 ± 6% and for CD8: 29 ± 10% versus 30 ± 4%). No significant differences were induced by HAART in the percentage of IL-2-expressing T cells (for CD4: 57 ± 11% at t3, 55 ± 13% at t6 and 55 ± 10% at t12; for CD8: 28 ± 8% at t3, 26 ± 10% at t6 and 27 ± 6% at t12). The absolute count of CD4+ IL-2 + T cells at baseline was 185 ± 90 cells/µl and rose to 263 ± 110 cells/µl (P = 0·001) at t3, to 312 ± 123 cells/µl (P = 0·007) at t6 and to 290 ± 72 cells/µl at t12 (P = 0·046). Similarly, the number of CD8+ IL-2 + T cells increased from the baseline value of 170 ± 67 cells/µl to 217 ± 76 cells/µl (P = 0·015) at t3, to 224 ± 84 cells/µl (P = 0·010) at t6 and to 204 ± 103 cells/µl (P = 0·42) at t12.
Single-cell analysis of IL-4-producing T cells
In HIV-1-infected patients the baseline frequency of CD4+, but not CD8+, IL-4-producing T cells was significantly reduced in comparison to normal controls (for CD4: 3·1 ± 2·5% versus 4·8 ± 1·3%, P = 0·03; for CD8: 2·8 ± 3% versus 1·6 ± 0·6%, P = 0·58, Fig. 1c). High levels of IL-4 production were observed in one patient within the CD4+ T cells (11%) and in another one within the CD8+ T cells (15%). HAART did not induce significant changes in the mean percentage of IL-4-producing cells within both CD4 and CD8 subsets (for CD4: 2·8 ± 2·6% at t3, 2·9 ± 2·6% at t6 and 2·4 ± 2·7% at t12; for CD8: 2·4 ± 3% at t3, 2·7 ± 3·6% at t6 and 1·8 ± 1·6% at t12) although in some patients an increase was observed (data not shown). The absolute number of IL-4-expressing cells (Fig. 1f) rose significantly only within the CD4 subset up to t6 (from 11 ± 13 cells/µl at baseline to 13 ± 13 cells/µl at t3, P = 0·02 and to 17 ± 15 cells/µl at t6, P = 0·01). Within the CD8 subset the number of IL4 + cells increased from 17 ± 26 cells/µl at baseline to 18 ± 20 cells/µl at t3, P = 0·67, 23 ± 26 cells/µl at t6, P = 0·36 and 14 ± 13 cells/µl at t12, P = 0·53. A correlation between the percentage of IL-4-producing cells and the percentage of memory, CD95+ and CCR5 + T cells was observed in CD4+ (r = 0·64, r = 0·75 and r = 0·50, respectively, and P < 0·0001 at all time points) but not in CD8+ cells. A significant correlation was also observed between the percentages of IL-4 and IFN-γ expressing T cells within the CD4+ (r = 0·9, P < 0·0001)
CTL activity
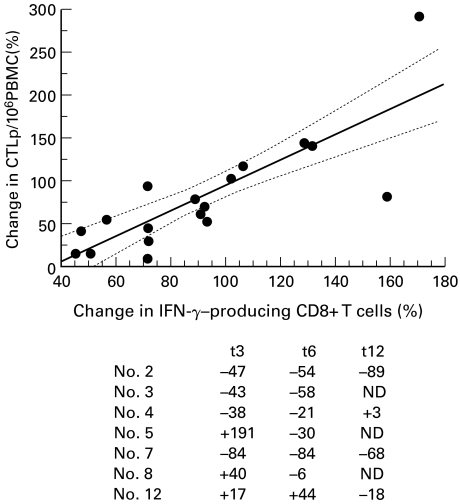
The frequencies of HIV Gag-e Pol-specific CTLp were estimated by limiting dilution assay before and during HAART in 7 patients randomly selected among the 20 HIV-1 infected individuals investigated in the present study (patients nos.2–5, nos.7,8 and no.12). Anti-HIV CTLp were detected, at baseline, in all patients investigated, ranging from 79 to 1142/106 PBMC. No meaningful correlations were observed between the number of CTLp and viral load, CD4+ and CD8+ counts. As shown in Fig. 2, a reduction in the frequency of anti-HIV CTLp was detected after therapy in 4/7 patients at t3 (57%) and in 6/7 at t6 (85%). In four out of 7 patients the peripheral distribution of CTLp has been assayed up to 12 months of therapy (namely patients no.2, no.4, no.7 and no.12). In patients (no.2, no.12) the level of plasma HIV-RNA measured at t12 did not differ from that determined at both t3 and t6, remaining under the level of detectability of the assay. The number of CTLp continued to decrease in patient no.2 and no.12, whose viral load remained undetectable, while it rose in patients no.4 and no.7 where a rebound of viral replication was seen. As shown in Fig. 2, the HAART-induced changes in frequency of CTLp significantly correlated with changes of CD8+ IFN-γ producing T cells (r = 0·82, P < 0·0001).
Fig. 2.
Correlation between changes in the proportion of anti-HIV Gag and Pol CTLp and IFN-γ-producing CD8+ T cells. Data are expressed as percentage of changes from baseline after 3, 6 and 12 months of HAART and referred to 7 out of 20 patients according to the numeration used in Table 1. The numbers in the table indicate the percentage change from baseline in the proportion of CTLp/106 PBMC at t3, t6, and t12, respectively.
Longitudinal study of TCRBV repertoire
The expression of 24 TCRBV subfamilies was firstly investigated in CD4+ and CD8+ T cells by cytofluorometry (Table 3). Results showed that the extent of skewedness in BV usage within CD4+ cells was significantly modified by 12 months of HAART: 46 expansions at t0 versus 30 at t3 (P = 0·71), 32 at t6 (P = 0·64) and 7 at t12 (P = 0·002). As opposite, in CD8+ T cells an initial significant increase in the number of BV expansions was seen at t3 (39 expansions at t0 versus 44 at t3, P = 0·018) followed by a decrease, at both t6 and t12, that did not reach statistical significance (39 expansions at t6 P = 0·11 and 16 expansions at t12, P = 0·99).
Table 3.
Expansions of variable chain beta (BV) subfamilies within the CD4 and CD8 T cell subsets. The expanded BV subfamilies are shown in bold whereas in brackets are indicated the relative percentages
| Patients | t0 | t3 | t6 | t12 |
|---|---|---|---|---|
| CD4+ | ||||
| 1 | 6·7 (19·7); 12 (4·9); 13·6 (8·8) | 6·7 (9); 12 (4·4); 13·6 (9·9) | 6·7 (16·3); 12 (4); 13·6 (9·6) | 13·6 (6·5) |
| 2 | 9 (8) | 9 (9·7) | 9 (10); 12 (7·8) | ND |
| 3 | 7 (6·9); 12 (4·1); 21·3 (4) | 3 (11·2) | 0 | 0 |
| 4 | 18 (5); 23 (1·5) | 11 (2·4); 16 (5); 23 (2) | 11 (2·2); 12 (5); 23 (1·5) | 0 |
| 5 | 2 (16); 7 (5); 13·1 (10·9); 21·3 (3·8) | 13·1 (13·7) | 5·2 (3·6); 13·1 (10) 21·3 (5·0) | 13·1 (6·1) |
| 6 | 5·1 (9·2); 13·1 (5·8); 14 (5·2); 16 (4·5) | 11 (2·5); 13·1 (5·7); 14 (5·4) | 16 (2·4); 21·3 (4·8); 23 (3·2) | 16 (2·1) |
| 7 | 16 (3·5); 23 (1·6) | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 |
| 9 | 0 | 11 (1·4); 21·3 (4·4) | 0 | 0 |
| 10 | 6·7 (8·8); 12 (4) | 12 (4·1); 16 (2·3) | 11 (2·8); 12 (4); 13·1 (8·7); 23 (2) | 0 |
| 11 | 11 (6·8) | 5·1 (8·6); 16 (2·9) | 5·1 (9); 11 (1·4); 12 (4·9); 16 (2·5) | ND |
| 12 | 11 (1·5); 16 (3) | 0 | 5·2 (2·9); 23 (14) | 0 |
| 13 | 6·7 (17·3) | 6·7 (17·7) | 6·7 (12·7) | 6·7 (14) |
| 14 | 9 (7·8); 11 (5·5); 23 (1·5) | 2 (16·8) | 0 | 2 (18·2) |
| 15 | 14 (5); 16 (2·8): 18 (4·3) | 7 (5·5); 17 (4·4); 21·3 (3·7) | ND | 0 |
| 16 | 8 (8·7); 11 (5·7); 12 (11); 17 (12·5) | 12 (7); 17 (13) | 8 (10); 12 (9); 14 (14); 17 (14) | OUT |
| 17 | 5·1 (9·7) | 5·1 (8·6); 16 (2·7) | 14 (5·7) | 0 |
| 18 | 5·1 (10·4); 13·1 (6·3); 21·3 (3·7); 23 (3·7) | 5·1 (9); 11 (1·7); 13·1 (6·5) | 0 | 5·2 (6·3); 13·6 (4·6) |
| 19 | 5·2 (7·9); 12 (5); 16 (3·9); 23 (2·5) | 0 | 11 (2·2); 17 (9·9) | 0 |
| 20 | 9 (7·6); 13·6 (6·9) | 0 | 0 | 0 |
| Total BV expansions | 46 | 30 (ns) | 32 (ns) | 7 (P = 0·0022) |
| CD8+ | ||||
| 1 | 14 (17·3); 16 (17) | 14 (17) | 14 (17·9) | 14 (21·2) |
| 2 | 2 (17); 9 (9); 18 (4); 22 (21) | 5·1 (4·6); 22 (28·2) | 2 (14·9); 22 (31·3) | ND |
| 3 | 5·1 (6); 11 (3·7) | 5·1 (4·4); 16 (5·7) | 0 | 0 |
| 4 | 5·2 (6·3); 11 (2·4); 18 (4·7) | 5·2 (6·7); 11 (2·5) | 5·2 (5·6); 12 (5); 16 (7·7); 20 (7·7) | ND |
| 5 | 14 (8·9); 16 (7·3); 20 (6·0) | 14 (9·4); 16 (8) | 5·1 (4·8); 11 (3·4); 14 (9); 16 (5·5); 23 (5·9) | 16 (6·1) |
| 6 | 0 | 16 (6·0) | 16 (9·1) | 16 (13·2) |
| 7 | 0 | 0 | 0 | 12 (4·9) |
| 8 | 16 (8) | 5·1 (8·9); 9 (6·2); 11 (3·2); 13·1 (7) | 11 (2·7) | 0 |
| 9 | 13·6 (3·4) | 16 (5·7) | 5·1 (4·9) | 16 (10·7) |
| 10 | 14 (8·2) | 13·1 (12·4); 14 (10·8); 16 (9·3) | 12 (9); 13·1 (10·5) | 13·1 (9·0) |
| 11 | 11 (7·6); 13·6 (3·4) | 5·1 (4·6); 17 (10·7) | 5·1 (5·9); 13·6 (3·1); 17 (9·3) | ND |
| 12 | 13·6 (2·9); 14 (9·9); 16 (10·9); 21·3 (6·2) | 21·3 (9·6) | 14 (8·5); 21·3 (9·5) | 14 (8·5); 22 (5·8) |
| 13 | 13·1 (6·6) | 3 (14·7); 13·1 (7·2); 14 (8·2) | 3 (13·3); 13·1 (8·7) | 3 (12·6); 13·1 (8·4) |
| 14 | 13·1 (13·5); 13·6 (3) | 13·1 (20); 13·6 (3) | 13·1 (18); 13·6 (3·2) | 13·1 (19·9); 13·6 (3·9) |
| 15 | 8 (11·8) | 3 (12·7); 8 (8·9); 18 (3·5) | ND | 0 |
| 16 | 8 (8·1); 11 (2·3) | 5·2 (3·6); 8 (9·5); 13·1 (6·4) | 8 (8·4); 23 (5·2) | OUT |
| 17 | 14 (9·3) | 13·1 (7); 14 (9); 19 (8·9) | 13·1 (7) | 0 |
| 18 | 5·1 (5); 13·6 (3·9); 16 (14) | 5·1 (5); 13·1 (8·4), 13·6 (4·5); 14 (21) | 13·1 (9·8); 13·6 (3·9); 14 (11·8) | 13·6 (3·8) |
| 19 | 11 (2·1); 12 (5·8); 23 (10·4) | 18 (3·1); 22 (12); 23 (6·9) | 5·1 (4·6); 13·6 (3·5); 22 (11); 23 (8·5) | 0 |
| 20 | 5·1 (5·8); 13·1 (10); 13·6 (10·6) | 13·1 (10·2); 13·6 (18·7) | 5·1 (4·4); 13·1 (11·3); 13·6 (15) | 13·1 (11·4); 13·6 (17·2); 16 (6·9) |
| Total BV expansions | 39 | 44 (P = 0·018) | 39 (ns) | 16 (ns) |
The number of TCRBV expansions within the CD4 subset was not significantly related to the CD4 count (P = 0·67). However, when patients were stratified according to the CD4+ T cell count, above or under 250 cells/µl, a significantly different number of expansions could be demonstrated in the two groups of patients (26 expansion in 7 patients under 250 cells/µl, mean 3·71, versus 21 expansions in 13 patients above 250 cells/µl, mean 1·61, P = 0·046). Expansions were observed in almost all BV families investigated and particularly within BV 11, 12 and 16 for CD4 and within BV 5·1, 13·1 and 16 for CD8.
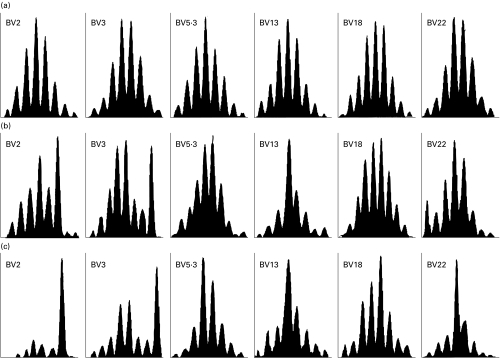
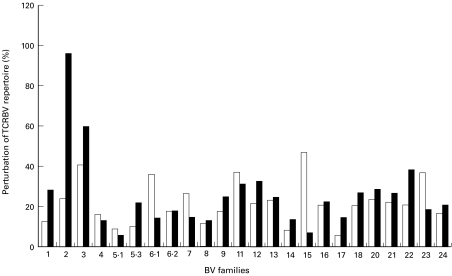
The CDR-3 fragment length analysis of 24 TCRBV subfamilies was also performed. As expected, perturbations of TCRBV repertoire were more frequently observed in HIV-1 infected individuals than in HIV-1 negative individuals. The “spectratypes” generated from CD4+ T cells before the onset of therapy showed a similar gaussian policlonal profile for the major part of BV families investigated. Perturbed CDR-3 profiles were observed, within the CD4 subset, only in patients with CD4 count <200 cells/µl. As opposite, all patients showed, within the CD8 subset, oligoclonal peaks in each TCRBV families studied. However, it should be noted that oligoclonal expansions are commonly observed, although to a lesser extent, also in CD8+ T cells from healthy individuals (data not shown). Perturbed CDR-3 profiles were not significantly modified by 12 months of HAART, both in CD4+ (Figs 3 and 4) and in CD8+ subset (data not shown).
Fig. 3.
CDR-3 size analysis of 6 representative CD4+ TCRBV families from (a) an healthy individual and from patient no.14 (b) before and (c) after 12 months of HAART.
Fig. 4.
CDR-3 perturbation, of 24 TCRBV family studied at t0 (▪) and t12 (□). Perturbation was calculated by subtracting the area under curve (AUC) of each CDR-3 peak detected in patient no.14 from the AUC of 5 HIV-negative donors.
DISCUSSION
In this report are shown the immunological correlates of HAART-induced suppression of HIV-1 replication occurring in asymptomatic patients naive for PI. The T cell response was characterized by an early rise of naive and memory cells both in CD4+ and CD8+ T cells. In previuos studies designed to characterize the HAART-induced T cell repopulation in patients with advanced HIV disease, an increase of CD4+ naive T cells was observed beginning from 6 to 12 months of therapy [2,3,23]. The faster increase in CD4+ naive cells observed herein could be explained by a lesser HIV-induced blockade of T cell renewal occurring in patients with moderate immunodeficiency as well as to a more preserved thymic tissue [24]. In addition, other mechanisms such as the peripheral expansion of naive lymphocytes or the reversion of phenotype from memory cells have been invoked to explain the increase of naive cells during HAART, as recently proposed by Zhang et al. [25] showing that the thymic output, evaluated by measuring the TCR excisional circles (TRECs), does not enterely account for the HAART-induced rise in naive cells.
The chemokine receptors CCR5 and CXCR4 are the major coreceptors for HIV-1 [26,27], mainly expressed on memory/activated and naive/unactivated T cells, respectively [28]. It has been suggested that the most significant variables influencing the efficiency of viral entry are both CD4 and coreceptors cell surface expression levels [29,30]. It is now well documented that the percentage of CCR5-expressing T cells increases as a consequence of disease progression. This is demonstrated by the correlation existing between high CCR5 expression and low CD4+ cell numbers, high HLA-DR expression and high viral RNA load in serum [13,31]. Herein we confirm, and extend, our recently reported data on CCR5 expression during HAART, showing a down regulation of this coreceptor on both CD4+ and CD8+ T cells following effective therapy [16]. The level of CXCR4-expressing T cells also decrease during HAART. Since the HAART-induced suppression of HIV replication is seen in association with a decreased expression of activation markers, we retain that the HAART-induced normalization of CCR5 expression on CD4+ T cells must be considered as a consequence of the reduced immune activation. However, this mechanism does not seem account for the observed changes in CXCR4 expression since it decreases during HAART in spite of decreased levels of immune activation. Moreover, our data apparently contrast with the reported enhancing effect exerted in vitro by IL-4 on CXCR4 expression [32]. In fact, patients with high levels of IL-4 producing cells show frequencies of CXCR4-expressing cells overlapping those of both normal controls and patients with normal IL-4 levels and no significant correlation was observed between frequency of IL-4-and CXCR4-expressing T cells. A possible explanation for this apparent discrepancy could probably reside in the in vivo concentrations of IL-4, not enough high to trigger CXCR4 expression.
Among the many immune abnormalities induced by HIV infection, an imbalance of cytokine production has been proposed to have a role in the pathogenesis of AIDS [8,9]. However, the suggested shift from a Th1 to Th2 cytokine production profile during the course of HIV-1 infection is still debated [33,34]. To this regard, previously reported data by Martinon et al. showed a stability of IL-2, IL-4 and IFN-γ mRNA levels during HAART [6]. Moreover, Sousa et al. described, more recently, a recovery in the frequency of IL-2-producing cells in patients with sustained control of viral replication [14]. Our data, showing a reduction in the frequency of CD8+ IFN-γ-producing T cells and an increased number of both CD4+ and CD8+ IL-2 producing T cells are consistent with the above mentioned reduction of immune activation and with ‘de novo’ generation of naive T cells. However, in some patients a ‘paradoxical’ increase in the frequency of IFN-γ-expressing cells was observed and this was seen in association with an increase in memory, Fas-and CCR5-expressing cells. We hypothesize that in these patients the increased frequency of activated-cells might be driven by residual HIV replication [35,36] or alternatively by a prevalent proliferation of cells with a memory phenotype. In any case, our results do not support the previously suggested theory of an imbalance in the pattern of cytokine producing T cells with a shift from a Th1 to Th2 cytokine production pattern [37].
It is well known that HIV disease progression associates with increasing disruptions in both CD4+ and CD8+ TCRBV repertoire [38]. We observed, at baseline, a significant perturbation of the TCR BV repertoire in both CD4+ and CD8+ subsets. After 12 months of HAART the number of TCR BV expansions in CD4+ cells was significantly reduced as indicated by the stratified data permutation analysis. In contrast, the diversity of the CD8 repertoire was furtherly diminished by 3 months of therapy and increased, but not significantly, only after 12 months of effective HAART. The correlation between low CD4 count and number of TCR BV expansions supports the concept that the decreased diversity of TCR repertoire is probably due to the CD4+ T cell depletion rather than to the supposed superantigen like activity exerted by HIV antigens [39]. Against this latter theory there is also the observation of no evident ‘holes’ in the TCR BV repertoire of HIV-1 infected individuals with less advanced disease.
Since the fine antigenic diversity of the TCR is almost entirely carried by the CDR3 region, we also performed the analysis of CDR3 size by PCR amplification across the VDJ junction. Oligoclonal profiles were observed in the major part of CD8+ TCR BV families, but rarely in CD4+ cells even in patients with low CD4+ counts. However, these oligoclonal expansions were relatively stable after therapy. This might have consequences on therapeutic intervention since an effective immune reconstitution is possible only whether new naive cells with a diverse repertoire are generated.
Our results showing a decreased number of Gag and Pol CTLp in patients with optimal CD4 and viral load responses to HAART support the notion that viral replication is essential to maintain detectable anti-HIV cytotoxic responses. The increased number of anti-HIV CTLp, observed in some patients after three months of HAART in spite of a dramatically inhibited HIV replication, could be related to the redistribution of CTLs from inflammed tissues toward the peripheral blood occurring during the early phase of T cell repopulation as also suggested by the increased perturbation of CD8+ TCR BV repertoire observed after the first 3 months of therapy. This hypothesis is further supported by the recently reported demonstration of the emergence, during the first month of therapeutic virus control, of non-HIV-specific T lymphocytes such as CMV-specific CD8+ T cells [40]. Alternatively, other mechanisms could be invoked such as proliferation of HIV-specific T cells or decreased apoptosis. Since the anti-HIV cytotoxic response probably represents the main component of the host's immune response involved in the control of HIV replication, the observed decline in the number of HIV-specific CTLp induced by HAART could hypotetically contribute to mechanisms of viral escape in case antiretroviral therapy fail. In this context, novel therapeutical strategies targeted to maintain efficient anti-HIV cytotoxic responses could be of relevance in allowing a long-term immunological response and, possibly, a reduction of antiretroviral drugs intake.
In conclusion, HAART-induced suppression of HIV-1 replication in patients with moderate immunodeficiency results in normalized levels of chemokine receptors expression, increased frequencies IL-2-producing T cells, decreased anti-HIV cititoxicity and significant improvement of CD4+ TCRBV repertoire. Ameliorated clinical conditions were also observed, even in those few patients who showed, in spite of successfully suppressed viral replication, a greater number of TCRBV expansions, worsened CDR3 profiles or increased frequency of activated T cells. These observations indicate that a better restoration of immunity may be obtained in patients starting HAART at less advanced stages of the disease.
Acknowledgments
This paper was supported by funding from the Istituto Superiore di Sanità, Italy, Grant n°40B.31998–99 and Progetti Ateneo University of Rome ‘La Sapienza’ 1998.
REFERENCES
- 1.Cavert W, Notermans DW, Staskus K, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–4. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 2.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 3.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 4.McCune JM, Hanley MB, Cesar D, et al. Factors influencing T-cell turnover in HIV-1-seropositive patients. J Clin Invest. 2000;105:R1–8. doi: 10.1172/JCI8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorochov G, Neumann AU, Kereveur A, et al. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–21. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Michelet C, Peguillet I, et al. Persistent alterations in T-cell repertoire, cytokine and chemokine receptor gene expression after 1 year of highly active antiretroviral therapy. Aids. 1999;13:185–94. doi: 10.1097/00002030-199902040-00006. [DOI] [PubMed] [Google Scholar]
- 7.Connors M, Kovacs JA, Krevat S, et al. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat Med. 1997;3:533–40. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 8.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 9.Romagnani S, Del Prete G, Manetti R, et al. Role TH1/TH2 Cytokines HIV Infection Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 10.Sabbaj S, Para MF, Fass RJ, Adams PW, Orosz CG, Whitacre CC. Quantitation of antigen-specific immune responses in human immunodeficiency virus (HIV)-infected individuals by limiting dilution analysis. J Clin Immunol. 1992;12:216–24. doi: 10.1007/BF00918092. [DOI] [PubMed] [Google Scholar]
- 11.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath MJ. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–74. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 12.Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind RA, Devaux C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268:329–44. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski MA, Justement SJ, Catanzaro A, et al. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998;161:3195–201. [PubMed] [Google Scholar]
- 14.Sousa AE, Chaves AF, Doroana M, Antunes F, Victorino RM. Kinetics of the changes of lymphocyte subsets defined by cytokine production at single cell level during highly active antiretroviral therapy for HIV-1 infection. J Immunol. 1999;162:3718–26. [PubMed] [Google Scholar]
- 15.Pontesilli O, Kerkhof-Garde S, Notermans DW, et al. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 16.Giovannetti A, Ensoli F, Mazzetta F, De Cristofaro M, Pierdominici M, Muratori DS, Fiorelli V, Aiuti F. CCR5 and CXCR4 chemokine receptor expression and beta-chemokine production during early T cell repopulation induced by highly active anti-retroviral therapy. Clin Exp Immunol. 1999;118:87–94. doi: 10.1046/j.1365-2249.1999.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell EB, Sparshott SM, Bunce C. CD4+ T-cell memory, CD45R subsets and the persistence of antigen – a unifying concept. Immunol Today. 1998;19:60–4. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- 18.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol. 1993;150:1122–36. [PubMed] [Google Scholar]
- 19.Carbonari M, Tedesco T, Del Porto P, Paganelli R, Fiorilli M. Human T cells with a type-2 cytokine profile are resistant to apoptosis induced by primary activation: consequences for immunopathogenesis. Clin Exp Immunol. 2000;120:454–62. doi: 10.1046/j.1365-2249.2000.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maslanka K, Piatek T, Gorski J, Yassai M. Molecular analysis of T cell repertoires. Spectratypes generated by multiplex polymerase chain reaction and evaluated by radioactivity or fluorescence. Hum Immunol. 1995;44:28–34. doi: 10.1016/0198-8859(95)00056-a. [DOI] [PubMed] [Google Scholar]
- 21.Pontesilli O, Klein MR, Kerkhof-Garde SR, Pakker NG, de Wolf F, Schuitemaker H, Miedema F. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J Infect Dis. 1998;178:1008–18. doi: 10.1086/515659. [DOI] [PubMed] [Google Scholar]
- 22.Schockmel GA, Yerly S, Perrin L. Detection of low HIV-1 RNA levels in plasma. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:179–83. doi: 10.1097/00042560-199702010-00013. [DOI] [PubMed] [Google Scholar]
- 23.Mezzaroma I, Carlesimo M, Pinter E, et al. Long-term evaluation of T-cell subsets and T-cell function after HAART in advanced stage HIV-1 disease. Aids. 1999;13:1187–93. doi: 10.1097/00002030-199907090-00006. [DOI] [PubMed] [Google Scholar]
- 24.Hazenberg MD, Otto SA, Stuart JW, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Lewin SR, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999;190:725–32. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 27.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–62. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 30.Doms RW, Peiper SC. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–90. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 31.de Roda Husman AM, Blaak H, Brouwer M, Schuitemaker H. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J Immunol. 1999;163:4597–603. [PubMed] [Google Scholar]
- 32.Jourdan P, Abbal C, Noraz N, et al. IL-4 induces functional cell-surface expression of CXCR4 on human T cells. J Immunol. 1998;160:4153–7. [PubMed] [Google Scholar]
- 33.Fakoya A, Matear PM, Filley E, et al. HIV infection alters the production of both type 1 and 2 cytokines but does not induce a polarized type 1 or 2 state. Aids. 1997;11:1445–52. doi: 10.1097/00002030-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Ledru E, Lecoeur H, Garcia S, Debord T, Gougeon ML. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J Immunol. 1998;160:3194–206. [PubMed] [Google Scholar]
- 35.Sharkey ME, Teo I, Greenough T, et al. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat Med. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dornadula G, Zhang H, VanUitert B, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. Jama. 1999;282:1627–32. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 37.Clerici M, Shearer GMA. TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 38.Cossarizza A. T-cell repertoire and HIV infection: facts and perspectives. Aids. 1997;11:1075–88. doi: 10.1097/00002030-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Westby M, Vaughan AN, Balotta C, Galli M, Clerici M, Dalgleish AG. Low CD4 counts rather than superantigenic-like effects account for differences in expressed T-cell receptor (TCR) repertoires between HIV-1 seropositive long-term non-progressors and individuals with progressive disease. Br J Haematol. 1998;102:1187–96. doi: 10.1046/j.1365-2141.1998.00912.x. [DOI] [PubMed] [Google Scholar]
- 40.Mollet L, Li TS, Samri A, et al. Dynamics of HIV-specific CD8+ T lymphocytes with changes in viral load. The RESTIM and COMET Study Groups. J Immunol. 2000;165:1692–704. doi: 10.4049/jimmunol.165.3.1692. [DOI] [PubMed] [Google Scholar]