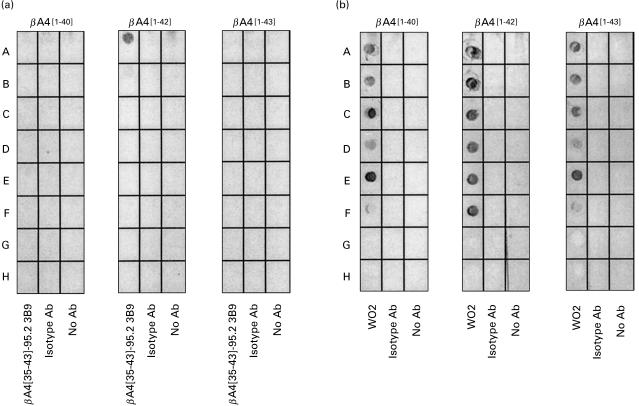
Fig. 7.
(a) Investigation of the stability of the βA4 38[GVV]40 epitope identified by MoAb 3B9 showed recognition of native βA4[1–42] but loss of this antigenic activity following heating and solubilization in 1% (w/v) SDS detergent. In this experiment, nitrocellulose was dotted with 1·0 μg of βA4[1–40], [1–42] or [1–43] and treated as follows: A, peptide dissolved in PBS; B, peptide dissolved in PBS and boiled for 5 min; C, peptide dissolved in 1% (w/v) SDS-non-reducing sample buffer; D, peptide dissolved in 1% (w/v) SDS-non-reducing sample buffer and boiled for 5 min; E, peptide dissolved in 1% (w/v) SDS-reducing sample buffer containing 25 mm DTT; F, peptide dissolved in 1% (w/v) SDS-reducing sample buffer containing 25 mm DTT and boiled for 5 min; rows G and H were not dotted with βA4 samples. These strips of nitrocellulose were then reacted with MoAb 3B9 (lane 1), isotype control ET-1 MoAb (lane 2) or PBS (lane 3) and bound mouse immunoglobulin detected as described for dot blotting above. (b) Reactivity of the WO2 MoAb [21] to each of the βA4 variants under identical conditions to those described for MoAb 3B9.