Abstract
Cognate interactions between CD154 (CD40 ligand, CD40L) on activated T cells and its receptor CD40 on various antigen-presenting cells are involved in thymus-dependent humoral immune responses and multiple other cell-mediated immune responses. We have studied the regulation of CD154 expression in human T cells after activation with anti-CD3 and anti-CD28 antibodies or after pharmacological activation of protein kinase C with phorbol 12-myristate 13-acetate, and the calcium ionophore ionomycin. Under these conditions, transcription of the CD154 gene was rapidly induced without requiring de novo protein synthesis. Pharmacological inhibitors of NF-κB activation down-regulated CD154 mRNA and protein levels. Cyclosporin A, an inhibitor of NF-AT activation, acted similarly, and the effects of both inhibitors were additive. A potential NF-κB binding site is present within the CD154 promoter at positions −1190 to −1181. In electrophoretic mobility shift assays, this sequence was specifically bound by NF-κB present in nuclear extracts from activated T cells. Furthermore, in transient co-transfection of Jurkat T cells, p65 activated the transcription of a reporter construct containing a multimer of this NF-κB binding site. These observations demonstrate a role of NF-κB transcription factors in the regulation of CD40L expression in activated primary human T cells.
Keywords: CD154, CD40 ligand, cyclosporin A, NF-κB, T cell activation
Introduction
Interactions between the receptor CD40 and its ligand (CD154 (CD40L)) play an important role in many immune responses. CD40 is a type I transmembrane protein belonging to the TNF receptor family and is detected on B cells early in ontogeny. CD40 is also synthesized by other cell types with a capacity to present antigen, e.g. monocytes and cells of the dendritic cell system including Langerhans' cells and follicular dendritic cells of secondary lymphoid organs. Thymic cortical and medullary epithelial cells, interdigitating cells, endothelial cells, the epithelial lining of various mucosal surfaces and the skin are also CD40 positive. CD40L is a 261 amino acid-long type II transmembrane protein with a molecular mass of 33 KDa and is a member of the tumour necrosis factor (TNF) family of cytokines. Its gene is preferentially expressed in activated CD4+ T cells and mast cells, but the surface protein can also be detected on monocytes, natural killer cells, CD8+ T-cells, activated eosinophils and basophils, and activated B-cells [1, 2].
Interaction between CD40 and CD40L mediates important signals that are required, in addition to cytokines, for proliferation of and for immunoglobulin class switching in B cells [3,4]. Consequently, prevention of interaction between CD40 and CD40L blocks in vitro activation of B cells by CD40L expressing T cells [5]. In line with this observation is the profound deficit in thymus-dependent humoral immunity in CD154-deficient mice [6,7] and in wild-type mice treated with anti-CD40L MoAb [8]. The pivotal role of the CD40/CD40L interaction in thymus-dependent humoral responses in vivo is further highlighted by the hyper-IgM syndrome, a rare X-linked immunodeficiency, which is characterized by mutations in the CD40L gene. This results in normal to high levels of IgM but absence of IgG, IgA and IgE classes of immunoglobulin in serum [8,9].
The functional consequences of the interaction of the ligand with CD40 on non-B cells are still incompletely elucideted. In addition to the up-regulation of co-stimulatory proteins on B cells [10,11], it has become well established that CD40–CD40L interactions contribute to the activation of co-stimulatory activity of several other types of antigen-presenting cells, in particular dendritic cells. Occupancy of the CD40 ectodomain on these cells up-regulates expression of adhesive and co-stimulatory proteins, e.g. CD58, CD80, CD86, ICAM-1 [12,13], and leads to dendritic cell production of cytokines such as TNF-α and IL-12 [12–14]. Furthermore, in mouse models of collagen-induced arthritis, experimental allergic encephalomyelitis, lupus nephritis, colitis and oophoritis, treatment with anti-CD40L monoclonal antibodies blocked development of the disease. In the case of established clinical experimental allergic encephalomyelitis, the disease could be cured this way [15,16]. In addition, there is preliminary evidence that dysregulated expression of CD40L may contribute to, or is at least associated with, certain autoimmune diseases in man. For instance, increased basal CD40L expression was seen on T cells in a subpopulation of patients suffering from active systemic lupus erythematosus. Moreover, upon activation, surface expression of CD40L on T cells from such patients is markedly higher and much more prolonged (until 48 h after stimulation) in comparison with normal controls. Finally, anti-CD40L MoAb can significantly inhibit the in vitro production of antinuclear autoantibodies by lymphocytes from active lupus patients [17].
The above observations indicate that CD40L gene expression is crucial in the initiation and progression of various immune responses. Within the mouse CD40L promoter, four potential NF-AT binding sites are present. An 18-bp oligonucleotide containing the most proximal NF-AT site was shown to bind NF-ATc and NF-ATp. However, a multimer of this 18 bp oligonucleotide coupled to an appropriate reporter plasmid did not mediate activation by NF-AT in transient transfection assays. In contrast, a longer multimer of 30 bp strongly stimulated transcription, and in electrophoretic mobility shift assays, the latter construct was shown to bind not only NF-ATp and NF-ATc but also AP-1. Therefore, it was suggested that the active NF-AT complex requires AP-1 [18]. This is consistent with other data showing that activation of protein kinase C and increased intracellular free Ca2+ concentration can induce membrane expression of CD40L. In the promoter region of the human CD40L gene two functional putative NF-AT binding sites were identified [19]. These sequences bind NF-ATp, suggesting that NF-ATp is also important in the induction of CD40L in human T cells [20]. However, several other binding sites are present within the human promoter that can potentially function as binding sites for other DNA-binding transcription factors [19].
Here, we report studies on the expression of CD154 (CD40L) in human T-cells from healthy human volunteers. The cells were stimulated ex vivo with PMA and the calcium ionophore ionomycin (PMA/ION), or with the combination of anti-CD3 and anti-CD28 MoAbs. Using flow cytometry and semiquantitative reverse-transcriptase PCR (RT-PCR), a strong induction of the CD40L protein and mRNA levels was seen, respectively. Notably, this induction was strongly inhibited in the presence of inhibitors of the NF-κB activation pathway. A potential NF-κB binding site (5′-AGGGATTTCC-3′) was identified in the promoter region between position −1190 and −1181 (Fig. 1). This region bound the NF-κB p65 homodimer and, to a weaker extent, the p65/p50 heterodimer. Finally, this site also mediated strong transcriptional activation of a reporter gene through overexpressed p65 and p65/p50 in transient transfections. Consequently, a role for NF-κB in the regulation of the human CD40L promoter is proposed.
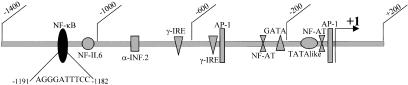
Fig. 1.
Map of the 5′ region in CD40L gene. The transcriptional start site is indicated with +1. Potential enhancers including NF-κB potential site are shown.
Materials and methods
Cell culture and stimulation
Blood was obtained by peripheral venipuncture of healthy adult volunteers. After isolation of peripheral blood mononuclear cells (PBMC) by density centrifugation over a Ficoll-Hypaque gradient (Histopaque, Sigma Chemicals; d = 1·007), T lymphocytes were further purified by negative selection using cold agglutination of monocytes and antibody-dependent complement-mediated lysis of B cells, monocytes and natural killer cells with Lympho-Kwik-T [21] (One Lambda, Los Angeles, CA, USA). T cells were cultured in RPMI 1640-Glutamax (Gibco Life Technologies, Scotland, UK) supplemented with 10% FCS, 1 mm non-essential amino acids, 1 mm sodium pyruvate, 50 mg/l of gentamycin and 10 mg/l of ofloxacin (Gibco), and activated with PMA (final concentration of 50 ng/ml) plus ionomycin (final concentration 1 µm); or with coated UCHT1 (anti-CD3ε) (ATCC, Rockville, MD, USA) plus soluble 9·3 (anti-CD28) (gift of Dr C. June, Abramson Cancer Research Center, Philadelphia, PA, USA) at a final concentration of 0·1 µg/ml. The following NF-κB inhibitors were used: the calpain 1 inhibitor N-acetyl-Leu-Leu-norleucinal (ALLN) (Boehringer Mannheim) in a final concentration of 50 µm; pyrrolidinedithiocarbamate (PDTC) (Sigma, St Louis, MO, USA) in a final concentration of 100 µm; Cyclosporin A (CsA) (Novartis, Basel, Switzerland), an inhibitor of calcineurin phosphatase, in a final concentration of 400 ng/ml; cycloheximide (Sigma), an inhibitor of protein synthesis a final concentration of 1 µg/ml; and Cbz-Ile-Glu(O-t-Bu)-Ala-Leucinal (PSI) (Bachem, Bubendorf, Switzerland), a specific proteasome inhibitor in a final concentration of 50 or 200 µm. Inhibitors were added to the cell cultures 1 h prior to activation. Cell viability was checked by trypan blue exclusion at the beginning and termination of cell cultures and always exceeded 95%.
Flow cytometry
Cells were suspended in 100 µl of PBS containing 1% w/w BSA (Sigma Chemicals). The cells were incubated for 30 min at 4°C with MoAb identifying the surface antigens of interest, or the proper isotype control MoAbs. FITC-conjugated anti-CD40L MoAb 24–31 (IgG1) was bought from Ancell. Anti-CD69 MoAb Leu-23 (IgG1) was purchased from Becton Dickinson. After two washes the cells were fixed with 1% w/V paraformaldehyde, and acquisition was performed on a FACSCAN flow cytometer (Becton Dickinson), with 488 nm as excitation wavelength and detection of fluorescence at 525 nm (FITC) or 590 nm (PE). Data were analysed with CellQuest software (Becton Dickinson).
RNA isolation and reverse-transcriptase (RT)-PCR
Total RNA from 107 T-lymphocytes was isolated by the guanidium isothiocyanate method [22]. cDNA was prepared from 2 µg of total RNA with the SuperScript (TM) reverse transcriptase preamplification system (Gibco Life Technologies) with oligo(dT) primer. Two µl of cDNA was used for the PCR. The CD40L specific primers were 5′-ACATACAACCAAACTTCTC CC-3′ (position +31 to +51, + 1 is ATG) and 5′-AGATGTTGTTTTACTGCTGGC-3′ (position +409 to +429) [19] which yielded a product of 400 bp. The reaction mix contained Goldstar Taq polymerase 12·5 U (Eurogentec), 1·5 mm MgCl2, 500 pmol of each primer and 200 µm of each dNTP in 125 µl total volume. This mixture was aliquoted in five different tubes (25 µl/tube) for PCR reactions in which the number of applied cycles was varied. The PCR conditions were: 30 s denaturation (94°C); 30 s annealing (60°C); 1 min extension (72°C); with an initial 5 min denaturation (94°C). PCR was terminated with addition of 2 µl of 0·5 m EDTA. The amplified products were resolved on a 1% agarose gel, containing ethidium bromide. CD69 was used as an internal control with the following primers: 5′-AAGTTCCTGTCCTGTGTGCTGTAA-3′ (position +112 to +135, + 1 is ATG), 5′-AATTCTTTGCCATTTGACC ACTTC-3′ (position +481 to +458). We performed RT-PCR of β-actin mRNA as a housekeeping gene control using the following primers: 5′-GAGGCCCAGAGCAAGAGAGGCATCC -3′ (human β-actin reverse primer, position +266 to +242); 5′-GCTCACCATGGATGATGATATCGCC-3′ (human β-actin forward primer; position +67 to +91).
Electrophoretic mobility shift assay
Purified T cells (107 cells in a final volume of 5–10 ml of complete culture medium) were stimulated with PMA (20 ng/ml) and ionomycin (1 µm) for 1 h, and nuclear extracts were prepared according to Dignam et al. [23]. The nuclear extract was resuspended in extraction buffer (20 mm Hepes pH 7·9, 0·4 m NaCl, 1 mm EDTA, 1 mm EGTA, 2·0 µg leupeptin/ml, 2·0 µg aprotinin/ml, 1 mm DTT and 1 mm PMSF) at a final concentration of 2·5 µg/µl. As a probe we used a double-stranded oligonucleotide encompassing the sequence of the NF-κB site of the CD154 (CD40L) promoter (− 1192 to −1174) [19]. The probe was labelled with γ32P-ATP using T4-polynucleotide kinase [24]. The HIV-LTR consensus NF-κB site and the endothelin GATA-2 site were used as specific and non-specific competitor DNA, respectively. The sequences of the upper strand of these oligonucleotides were as follows: CD40L: 5′-TGAGGTAGGGA TTTCCACAGCTG-3′; HIV-LTR: 5AGTTGAGGGGACTTTCC CAGGC-3′; GATA-2: 5′-AGCTCGGGCCTGGCCTTATCTCCG GCTGCACG-3′.
A total of 0·5 fmol of labelled oligonucleotide was added to 20 µl (final volume) reaction mixture containing 25 mm HEPES pH 7·9, 5% glycerol, 0·5 mm EDTA, 0·5 mm DTT, 50 mm NaCl, 1% NP-40, 2 µg poly (dI-dC). Unlabelled competitor DNA was added at 100-fold excess. Finally, the protein extract (5 µg) was added and the reaction was allowed to proceed for 15 min at 24°C. For the supershift assays, the extracts were incubated with antip65 antibodies or antip50 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 min at 24°C prior to binding of the probes. Samples were loaded immediately onto a 6% polyacrylamide gel made in Tris-Glycine-EDTA buffer. The gel was fixed, dried, and analysed by PhosphorImager (Molecular Dynamics).
Transfections of Jurkat cells
The 6 NF-κB CD40L/TKmin reporter was obtained by cloning six copies of the NF-κB binding site (5′-AGGGATTTCCA-3′) of the CD40L promoter upstream of the minimal thymidine kinase (TK) promoter in the pGL3 luciferase reporter plasmid. The pRc/CMV-p65, pRc/CMV-p50 and IκBα expression plasmids for p65, p50 NF-κB subunits and IκBα, respectively, were kindly provided by S. Plaisance (Department of Molecular Biology, University of Gent) and M. Karin (University of California at San Diego, CA, USA).
Transfections of the Jurkat T cell leukaemic line were carried out with diethylaminoethyl-dextran (DEAE-dextran) [24]. Ten µg of the reporter construct, 1 µg of each expression plasmid and 1 µg of CMV-β-gal plasmid was prepared in a transfection mixture consisting of 500 µl Tris-buffered-saline-Dextrose (pH 7·4), 40 µl DEAE -dextran (50 mg/ml) and 9·4 ml RPMI 1640. The cells were added to the mix and incubated for 20 min, 37°C, 5% CO2, after which the suspension was washed in RPMI with 15 U heparin. The cells were kept overnight in complete medium at 37°C in the presence or absence of inhibitors. The following day the cells were stimulated with PMA (50 ng/ml) plus ionomycin (1 µm) for 4 h. Luciferase activity was measured according to the manufacturer's protocol (Promega). Transfection efficiency was normalized by measuring β-galactosidase activity with Galacto-Light Plus (Tropix).
Results
Cyclosporin A (CsA) and inhibitors of NF-κB activation inhibit expression of CD40L levels on the surface of activated T cells
In order to assess involvement of NF-κB in the regulation of CD40L expression, T cells were activated in the presence versus absence of acknowledged antagonists of NF-κB activation, e.g. Cbz-Ile-Glu(O-t-Bu)-Ala-Leucinal (PSI) [25], N-acetyl-Leu-Leu-norleucinal (ALLN) [26] or pyrrolidinedithiocarbamate (PDTC) [27,28]. Both PSI, a specific inhibitor of the chymotrypsin-like activity of the proteasome [29], and ALLN, another inhibitor of the proteasome pathway [26], prevent degradation of I-κB, which eventually results in a lack of translocated NF-κB in the nucleus. The generation of cytoplasmic reactive oxygen intermediates, in particular OH° radicals, is a crucial event in the activation of NF-κB. Therefore, we also used PDTC, a scavenger of free radicals, and known to interfere with the activation of genes requiring NF-κB [27,28].
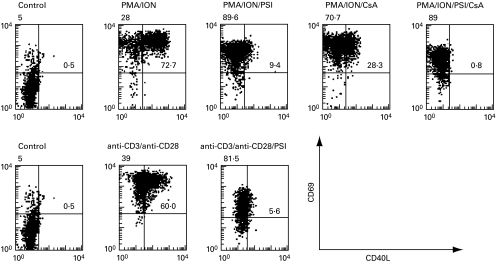
PMA/ION strongly and rapidly induced surface expression of CD40L and CD69 on T cells compared to non-treated T cells, as measured by flow cytometry (Fig. 2, upper panel) CD40L induction by PMA/ION was profoundly inhibited by PSI. In contrast, the CD69 level was only slightly affected. In the presence of PDTC and ALLN, the expression of CD40L was also inhibited (data not shown). CsA also inhibited CD40L levels, albeit to a lesser extent, while CD69 levels were not affected (Fig. 2, upper panel). Finally, the combination of CsA and PSI totally abolished the increase of CD40L surface levels, while CD69 was again much less affected. As a more physiological means to induce T cell activation, we also stimulated purified T cells with a combination of anti-CD3 and anti-CD28 MoAbs (Fig. 2, lower panel). Under these conditions, high surface levels of CD40L were observed after 24 h of stimulation, which were strongly inhibited by PSI. Similar results for CD40L were obtained with ALLN and PDTC (data not shown). The down-regulation of CD40L expression by CsA and by various NF-κB inhibitors suggest that both NF-AT and NF-κB, respectively, participate in the induction of CD40L expression in activated T cells.
Fig. 2.
PSI and CsA inhibit CD40L surface expression on activated T cells. T cells were stimulated with PMA (50 ng/ml) and ionomycin (1 µm) for 6 h (upper panel) or with immobilized anti-CD3 MoAb (UCHT1) and soluble anti-CD28 MoAb (9·3) (100 ng/ml) for 24 h (lower panel). PSI (200 µm) and/or CsA (400 ng/ml) were added as indicated. After the respective incubation time, the surface levels of CD40L and CD69 on the T cells were analysed by flow cytometry, following immunostaining with anti-CD40L MoAb and anti-CD69 MoAb. Dot plots of reactivity with FITC-conjugated anti-CD40L (x-axis) versus PE-conjugated anti-CD69 (y-axis) are shown.
Expression of CD40L mRNA in activated T cells does not require de novo protein synthesis and is inhibited by CsA and inhibitors of NF-κB activation
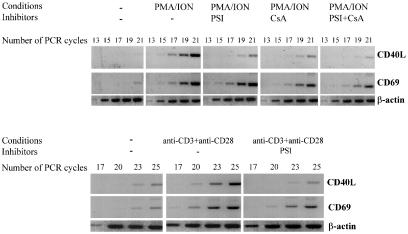
To investigate whether these inhibitors have a direct effect on the steady-state levels of CD40L mRNAs in activated T cells, a semiquantitative RT-PCR method was used. In unstimulated T cells, an amplified CD40L-specific DNA fragment could be detected only after 23 cycles of amplification (Fig. 3). When T cells were stimulated for 4 h with PMA/ION, the product from the RT-PCR was visible after 15 cycles of amplification, which indicates an approximately 1000-fold increase of CD40L mRNA expression. In the presence of PSI, the product was only visible after 19 cycles, indicating that PSI inhibits the induction of CD40L transcription about 16-fold upon stimulation with PMA/ION. Similar results were obtained when levels of CD40L mRNA were assayed in PMA/ION activated T cells in the presence of ALLN and PDTC, other NF-κB inhibitors. In the presence of CsA the amplified fragment was only detected after 19 cycles, again suggesting a 16-fold inhibition. The combination of PSI with CsA additively inhibited CD40L mRNA expression (21 amplification cycles needed). CD69 mRNA was also detected after stimulation with PMA/ION. As expected from the FACS analysis, the induction level of CD69 mRNA was much less affected by either PSI or CsA. Parallel RT-PCR showed quasi equal amounts of β-actin mRNA in all the RT-PCR samples, as shown in Fig. 3 (upper and lower panel).
Fig. 3.
PSI and CsA inhibit expression of CD40L mRNA in activated T cells. Purified resting T cells were stimulated with PMA (50 ng/ml) and ionomycin (1 µm) for 4 h or with immobilized anti-CD3 MoAb UCHT1 together with soluble anti-CD28 MoAb 9·3 (100 ng/ml) for 6 h. PSI (200 µm) and/or CsA (400 ng/ml) were added as indicated. After incubation, cDNA was synthesized and analysed by RT-PCR using primers specific for CD40L, CD69 or β-actin. The PCR products were separated in a 1% agarose gel, and analysed by staining with ethidium bromide.
When T cells were stimulated with a combination of anti-CD3 and anti-CD28 MoAbs, an eight-fold stimulation of CD40L mRNA expression was found after 6 h. In the presence of PSI, stimulation with anti-CD3 and anti-CD28 MoAbs failed to increase CD40L mRNA levels, while induction of CD69 mRNA expression was less affected. Finally, cycloheximide did not inhibit the induction of CD40L mRNA in T cells stimulated by PMA/ION, as shown previously [30], or by anti-CD3 plus anti-CD28 MoAbs (data not shown). This indicates that de novo protein synthesis is not required for the induction of CD40L mRNA expression, further supporting a role of NF-κB-like transcription factors in this induction.
We conclude that both PSI and CsA preferentially and not exclusively inhibit CD40L mRNA expression in activated T cells. Therefore, the reduced surface levels of CD40L in the presence of inhibitors of NF-κB correlate well with a decreased transcription of CD40L.
Identification of an NF-κB binding site within the CD40L promoter
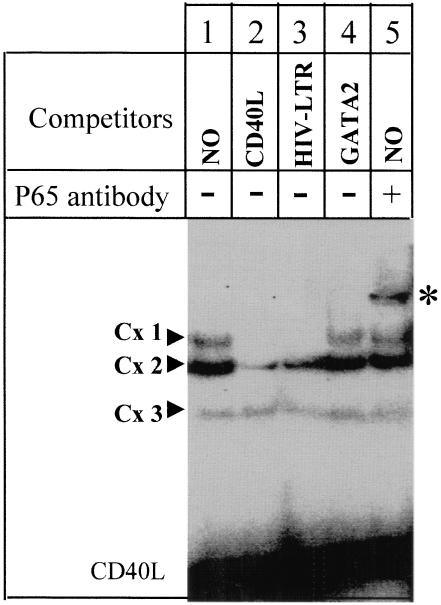
Upon screening of the promoter region of the human CD40L gene, we identified a potential NF-κB binding site (5′-AGGGATTTCC-3′) at positions (− 1190 to −1181) (Fig. 1). To test whether this sequence can bind NF-κB, we performed gel retardation experiments using as a probe an oligonucleotide corresponding to the promoter sequence (− 1196 to −1177). When this probe was incubated with a nuclear protein extract prepared from activated T cells, three protein : DNA complexes were identified (Cx1, Cx2 and Cx3) (Fig. 4, lane 1). Only Cx1 could be competed completely in the presence of an excess of unlabelled probe (lane 2). In addition, the Cx1 complex formation was totally competed by an excess of an oligonucleotide containing the NF-κB binding site of the HIV-long-terminal-repeat (lane 3), but was not affected by an excess of unrelated probe (GATA-2 binding site) (lane 4). This suggests that the factor(s) generating Cx1 bind(s) specifically to the CD40L probe and to the NF-κB site of the HIV-LTR. Cx2 was only competed partially with the CD40L probe and much less with the HIV-LTR and GATA-2 probes. This suggests that the protein generating the Cx2 complex binds specifically to the CD40L probe but is different from NF-κB because competition is weaker with the HIV-LTR NF-κB site. Cx3 is a non specific complex because it cannot be competed by any of the probes. To further identify the proteins present in Cx1, we preincubated the nuclear extract with a MoAb specific for the NF-κB subunit p65. This resulted in the formation of a supershifted complex, indicating that p65 is part of this retarded complex (lane 5). No supershifted complex was observed in the presence of p50 specific antibody or GATA-2 specific antibody (data not shown). We conclude that the CD40L promoter sequence between positions −1190 and −1181 can be bound by NF-κB transcription factors.
Fig. 4.
p65 binds to the CD40L promoter. fifty fmol of [32P]-labelled CD40L probe was incubated with nuclear extract prepared from activated T cells (lane 1). A 100-fold excess of either CD40L (lane 2), HIV-LTR (lane 3) or GATA-2 (lane 4) double-stranded unlabelled probe were added as competitor. In lane 5, the nuclear extract was incubated with antip65 NF-κB subunit antibody prior to the addition of the probe.
P65 or p65/p50 activates transcription via the CD154 (CD40L) NF-κB binding site
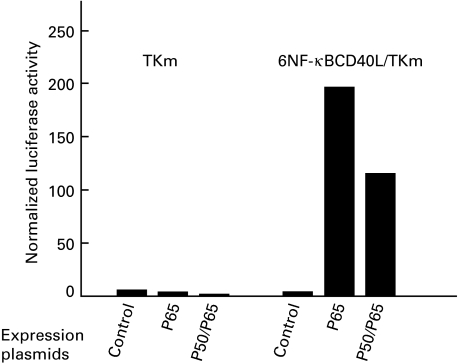
To investigate further whether p50 and p65 can activate transcription of CD40L via this NF-κB promoter element, we constructed a reporter vector containing six copies of the CD40L NF-κB oligonucleotide inserted upstream of the thymidine kinase minimal promoter into the luciferase pGL3 reporter plasmid (plasmid 6NF-κBCD40L/TKm). We performed transient co-transfection experiments in activated Jurkat cells using this reporter construct and expression vectors for p65 and/or p50 (Fig. 5). The minimal TK pGL3 reporter plasmid (TKm) and the empty expression vector were used as controls. Upon expression of p65, a strong transcriptional induction of the CD40L NF-κB reporter was observed, whereas the activity of the control reporter did not increase. When both p50 and p65 were expressed, we also observed an induction of the CD40L NF-κB reporter. This indicates that the CD40L NF-κB site can be activated via p65 homodimer as well as by p50/p65 heterodimers.
Fig. 5.
p65 and p65/p50 stimulate the expression of a reporter vector containing six copies of the CD40L NF-κB binding site. A hexamer of the CD40L NF-κB binding site was placed upstream of the TKm promoter, in the luciferase pGL3 reporter plasmid. p65 and p50 expression plasmids were co-transfected with this reporter plasmid into Jurkat cells; 20 h after the transfection, Jurkat cells were stimulated with PMA (50 ng/ml) and ionomycin (1 µm). The experiment was performed in triplicate and the intra-assay variation was less than 20%.
The NF-κB inhibitor PSI and overexpression of IκBα inhibit CD40L NF-κB reporter induction in Jurkat cells
PSI is a known inhibitor of NF-κB activation that has been shown here to down-regulate CD40L mRNA production. We wanted to assess whether PSI could affect transcription mediated by the NF-κB site that we identified in the CD40L promoter. To test this, we again transiently transfected the CD40L NF-κB plasmid reporter in Jurkat cells. The minimal TKpGL3 reporter was used as a negative control, and the NF-κB HIV pGL3 reporter, which contains six copies of the well characterized NF-κB site from HIV, was used as a positive control.
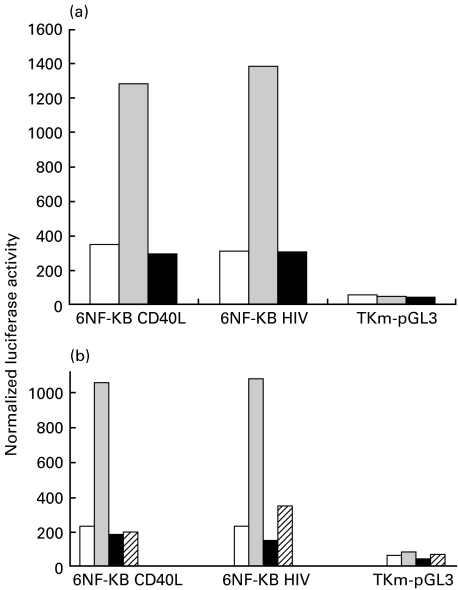
Upon stimulation whith PMA/ION, the luciferase activity from both the CD40L and HIV based NF-κB reporters increased about four-fold. This increase was completely blocked by addition of PSI to the activated cells, whereas there was no effect in the TKm reporter (Fig. 6a). We also transiently transfected Jurkat cells with the same reporter constructs together with an expression vector encoding IκBα, a physiological inhibitor of NF-κB. As control, the empty pcDNA3 expression plasmid was used. The minimal TKmpGL3 reporter was again used as reporter control. Upon stimulation with PMA/ION, the luciferase activity in Jurkat cells transfected with CD40L and HIV-based NF-κB reporters increased at least four-fold in the presence of the empty pcDNA3 plasmid. In contrast, when IκBα was overexpressed, activation of CD40L and HIV-based NF-κB reporters was markedly reduced (Fig. 6b). These observations further confirm that transcriptional activation of the CD40L NF-κB reporter is mediated by NF-κB.
Fig. 6.
The NF-κB inhibitor PSI and overexpression of IκBα inhibits the CD40L NF-κB reporter induction in Jurkat cells. (a) Jurkat cells were transfected with 6NF-κB CD40L, 6NF-κB HIV or TKm pGL3 reporter vector. Transfection mixtures were split and added into three different wells. Twenty hours after transfection, samples were either left unstimulated (NS), stimulated with PMA (50 ng/ml) and ionomycin (1 µm) (S), or stimulated with PMA (50 ng/ml) and ionomycin (1 µm) in the presence of PSI (50 µm) (S + PSI). Results are representative of three independent transfections. NS □; S ▪; S + PSI  . (b) Jurkat cells were co-transfected with 6NF-κB CD40L, 6NF-κB HIV or TKm pGL3 reporter vectors along with the expression vector for IκBα or the empty pcDNA3 expression plasmid. Transfection mixtures were split into two different wells. Twenty hours after transfection, samples were either left unstimulated (NS), or stimulated with PMA (50 ng/ml) and ionomycin (1 µm) (S). Results are representative of three independent transfections. NS + pcDNA3 □; S + pcDNA3
. (b) Jurkat cells were co-transfected with 6NF-κB CD40L, 6NF-κB HIV or TKm pGL3 reporter vectors along with the expression vector for IκBα or the empty pcDNA3 expression plasmid. Transfection mixtures were split into two different wells. Twenty hours after transfection, samples were either left unstimulated (NS), or stimulated with PMA (50 ng/ml) and ionomycin (1 µm) (S). Results are representative of three independent transfections. NS + pcDNA3 □; S + pcDNA3  ; NS + IκBα ▪; S + IκBα.
; NS + IκBα ▪; S + IκBα.
Discussion
Acquisition of CD154 (CD40L) on the surface of T cells occurs as an early event after activation. However, the mechanisms underlying the control of the CD40L gene expression remain poorly documented. We and others [30] have shown that CD40L mRNA induction is not inhibited by cycloheximide, indicating that de novo protein synthesis is not required. First of all, this indicates that CD154 (CD40L) gene expression, upon T cell activation, does not depend on synthesis of intermediate cytokines. Secondly, this also suggests that immediate transcription factors, e.g. NF-κB, can play a role in CD40L gene activation. This is further supported by our finding that inhibitors of NF-κB activation inhibit the up-regulation of CD40L protein levels and mRNA levels during early T cell activation by PMA/ION or the combination of anti-CD3 and anti-CD28 MoAbs. We have not ruled out decreased mRNA stability in the presence of the inhibitors used. However, these inhibitors all inhibit NF-κB, but by distinct mechanisms. Moreover NF-κB is not known to increase mRNA stability. It seems unlikely that besides inhibition of NF-κB, all three compounds would share the property of decreasing mRNA stability.
A potential site (5′-AGGGATTTCC-3′) was identified at positions −1191 to −1182 in the human CD40L promoter [18]. This site differs from the NF-κB consensus site (5′-GGGPuNNPyPyCC-3′) by the fact that the first G is not conserved. However, other sites in which this first G is absent, have previously been shown to bind p65 homodimer with an equal affinity as the consensus NF-κB site, but fail to bind p50 homodimers [31]. Using nuclear extracts from activated T cells in gel retardation assays, we identified a specific DNA-protein complex (named Cx1) with the CD40L NF-κB site. A strong supershift of the this complex was obtained with the antip65 antibody, but not with antip50. Furthermore, when this site was tested in co-transfection with a p65 expression plasmid, or p65 and p50 expression plasmids, we observed a strong activation. Finally, transcriptional activation mediated by this site was inhibited by PSI and by overexpression of IκBα. These results all point towards a role for NF-κB in the regulation of CD40L gene expression.
CD69 is another T cell activation marker which is rapidly induced following stimulation with PMA/ION or with anti-CD3 plus anti-CD28 MoAbs. However, unlike CD40L, CD69 protein and mRNA expression was much less markedly decreased in the presence of NF-κB inhibitors, as has also been reported by others [32]. Taken together, our data point towards a role for NF-κB transcription factors in the regulation of CD40L gene expression, and illustrate that NF-κB transcription factors are not invariably involved in the regulation of all early T cell activation markers.
Four NF-AT consensus sites have previously been mapped within the mouse CD40L promoter. An 18-bp oligonucleotide containing the proximal site was shown to bind NF-ATc and NF-ATp. A multimer of this proximal site was not active in transient transfection assays. In contrast, a longer 30 bp oligonucleotide, which was shown to bind both NF-AT and AP-1, strongly induced reporter gene expression. In the presence of CsA, the surface expression and mRNA expression of CD40L were down-regulated as well. This supports a role for NF-AT-like transcription factors in the regulation of human CD40L expression [20]. In fact, the combination of CsA and PSI had an additive effect, as they almost completely blocked the induction of CD40L gene expression in activated human T cells. This suggests that NF-AT and NF-κB transcription factors act cooperatively to induce CD40L expression, as is the case for several other genes induced upon T cell activation. Consistent with these finding T-cells from NF-κB/p50-deficient mice have reduced expression of CD40L, as did T cells from wild-type mice in the presence of proteasome inhibitors [33].
In general, our studies identify NF-κB a candidate transcription factor that is involved in the regulation of CD40L gene expression. A more complete understanding of the expression of this gene, and its modulation by NF-AT and NF-κB, may open up new possibilities to interfere with multiple types of pathological T cell dependent immune responses via the CD40L promoter as target. Conversely, immunosuppressive strategies using anti-CD154 MoAb may have to take into account the effects of immunosuppressive agents with impact on NF-AT or NF-κB activation.
Acknowledgments
This research was supported by grants 1.5.994.95 and G.156.96 of Fonds voor Wetenschappelijk Onderzoek (FWO)-Vlaanderen to P.V. P.V. is a postdoctoral fellow of FWO-Vlaanderen; S.P. was supported by a research fellowship of the Flemish Institute for the Promotion of Scientific/Technological Research in Industry (IWT), Brussels. J.E.R. and D.H. were supported by VIB. M.S. is recipient of a stipend from the Sophea-CRUH foundation. We thank J.L. Ceuppens for critical reading of the manuscript, S. Plaisance (Department of Molecular Biology, University of Gent) for providing us with NF-κB expression plasmids and C.H. June, Abramson Cancer Research Center, University of Pennsylvania, USA for generously providing anti-CD28 MoAb 9.3. The technical assistance of W. Scheers in flow cytometry is greatly acknowledged.
References
- 1.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 2.Noelle RJ. CD40 and its ligand in host defense. Immunity. 1996;4:415–9. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle PA, Baltimore D, Collart MA, Baeuerle P, Vassalli P. NF-kappa B: ten years after regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Cell. 1996;87:13–20. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grewal IS, Flavell RA. The CD 40 ligand. At the center of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 5.Noelle RJ, Ledbetter JA, Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992;13:431–3. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- 6.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–20. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 7.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 8.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E, Noelle RJ. gp39–Cd40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–63. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyper–IgM syndrome. Immunol Today. 1993;14:559–64. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 10.Ranheim EA, Kipps TJ. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177:925–35. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claasen E, Noelle RJ. gp39–Cd40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–63. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cella M, Scheidegger D, Palmer Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: up-regulation via MHC class II and CD40 molecules and down-regulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerritse K, Laman JD, Noelle RJ, et al. CD40–CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:2499–504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc Natl Acad Sci USA. 1995;92:4342–6. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai Mehta A, Lu L, Ramsey Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97:2063–73. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsytsykova AV, Tsitsikov EN, Geha RS. The CD40L promoter contains nuclear factor of activated T cells-binding motifs which require AP-1 binding for activation of transcription. J Biol Chem. 1996;271:3763–70. doi: 10.1074/jbc.271.7.3763. [DOI] [PubMed] [Google Scholar]
- 19.Shimadzu M, Nunoi H, Terasaki H, et al. Structural organization of the gene for CD40 ligand: molecular analysis for diagnosis of X-linked hyper-IgM syndrome. Biochim Biophys Acta. 1995;1260:67–72. doi: 10.1016/0167-4781(94)00179-7. [DOI] [PubMed] [Google Scholar]
- 20.Schubert LA, King G, Cron RQ, Lewis DB, Aruffo A, Hollenbaugh D. The human gp39 promoter. Two distinct nuclear factors of activated T cell protein-binding elements contribute independently to transcriptional activation. J Biol Chem. 1995;270:29624–7. doi: 10.1074/jbc.270.50.29624. [DOI] [PubMed] [Google Scholar]
- 21.Vandenberghe P, Ceuppens JL. Immobilized anti-CD5 together with prolonged activation of protein kinase C induce interleukin 2-dependent T cell growth: evidence for signal transduction through CD5. Eur J Immunol. 1991;21:251–9. doi: 10.1002/eji.1830210203. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Dignam JD. Preparation of extracts from higher eukaryotes. Meth Enzymol. 1990;182:194–203. doi: 10.1016/0076-6879(90)82017-v. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook T, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. Embo J. 1995;14:2876–83. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read MA, Neish AS, Luscinskas FW, Palombella VJ, Maniatis T, Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995;2:493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 27.Ferran C, Millan MT, Csizmadia V, et al. Inhibition of NF-kappa B by pyrrolidine dithiocarbamate blocks endothelial cell activation. Biochem Biophys Res Commun. 1995;214:212–23. doi: 10.1006/bbrc.1995.2277. [DOI] [PubMed] [Google Scholar]
- 28.Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: aP-1 as secondary antioxidant-responsive factor. Embo J. 1993;12:2005–15. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traenckner EB, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. Embo J. 1994;13:5433–41. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suarez A, Mozo L, Gayo A, Zamorano J, Gutierrez C. Requirement of a second signal via protein kinase C or protein kinase A for maximal expression of CD40 ligand. Involvement of transcriptional and posttranscriptional mechanisms. Eur J Immunol. 1997;27:2822–9. doi: 10.1002/eji.1830271112. [DOI] [PubMed] [Google Scholar]
- 31.Kunsch C, Ruben SM, Rosen CA. Selection of optimal kappa B/Rel DNA-binding motifs: interaction of both subunits of NF-kappa B with DNA is required for transcriptional activation. Mol Cell Biol. 1992;12:4412–21. doi: 10.1128/mcb.12.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellanos MD, Munoz C, Montoya MC, LaraPezzi E, Lopez-Cabrera M, de Landazuri MO. Expression of the leukocyte early activation antigen CD69 is regulated by the transcription factor AP-1. J Immunol. 1997;159:5463–73. [PubMed] [Google Scholar]
- 32.Roy M, Aruffo A, Ledbetter J, Linsley P, Kehry M, Noelle R. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur J Immunol. 1995;25:596–603. doi: 10.1002/eji.1830250243. [DOI] [PubMed] [Google Scholar]
- 33.Smiley ST, Csizmadia V, Gao W, Turka LA, Hancock WW. Differential effects of cyclosporine A, methylprednisolone, mycophenolate, and rapamycin on CD154 induction and requirement for NFkappaB: implications for tolerance induction. Transplantation. 2000;70:415–9. doi: 10.1097/00007890-200008150-00005. [DOI] [PubMed] [Google Scholar]