Abstract
We have reported previously that the abnormally down-regulated protein kinase C (PKC) causes cellular dysfunction observed in natural killer (NK) cells, polymorphonuclear leucocytes (PMNs) and fibroblasts from beige mouse, an animal model of Chediak–Higashi syndrome (CHS). Here we show that the abnormal down-regulation of PKC activity also occurs in Epstein–Barr (EB) virus-transformed cell lines from CHS patients. When CHS cell lines were stimulated with concanavalin A (Con A) for 20 min, the membrane-bound PKC activity declined markedly, whereas that in control cell lines increased. We found that E-64-d, which protects PKC from calpain-mediated proteolysis, reversed the declined PKC activity and corrected the increased Con A cap formation to almost normal levels in CHS cell lines. We confirmed that the dysregulation of PKC activity also occurred in peripheral blood mononuclear leucocytes (PBMC) from CHS patients and that E-64-d corrected both the declined PKC activity and increased Con A cap formation. E-64-d also corrected the reduced lysosomal elastase and cathepsin G activity in CHS cell lines. In contrast, chelerythrin, a specific inhibitor of PKC, and C2-ceramide, which promotes PKC breakdown induced by calpain, increased Con A cap formation and inhibited both elastase and cathepsin G activity in normal cell lines. Moreover, we found that ceramide production in CHS cell lines increased significantly after Con A stimulation, which coincides with our previous observation in fibroblasts from CHS mice. These results suggest an association between ceramide-induced PKC down-regulation and the cellular dysfunctions in CHS.
Keywords: calpain, ceramide, Chediak–Higashi syndrome, lysosome, protein kinase C
Introduction
Chediak–Higashi syndrome (CHS) is a rare, autosomal recessive disorder characterized by partial albinism, defective natural killer (NK) activity and abnormal intracellular protein transport to, and from the lysosome [1–4]. There is no specific treatment, and most patients succumb to frequent bacterial infections and an associated lymphoproliferative disorder. Many patients with CHS die young, unless they undergo bone marrow transplantation.
The beige mouse has been used as an animal model of CHS. The clinical and pathological features of CHS and beige mouse are very similar. Recently, the human CHSCI and beige genes were cloned, and pathological gene mutations were identified by two groups [5–7]. The sequence of human cDNA is homologous to beige mouse, and bg maps on proximal mouse chromosome 13 within a linkage group conserved with human chromosome 1q42–q43 [8]. The mutations of CHSCI gene were identified in CHS patients [9].
Protein kinase C (PKC) is a Ca2+, phospholipid-dependent, serine/threonine protein kinase and plays an essential role in intracellular signal transduction for various cell functions [10]. It is known that phorbol esters such as phorbol 12-myristate 13-acetate (PMA) activate PKC and cause a translocation of PKC from the cytosolic to the membrane fraction [11,12]. After the translocation, PKC is known to be proteolysed by calpain, which is a Ca2+-dependent thiol proteinase, to an inactive form [13]. We have reported previously that PKC activity was abnormally down-regulated in NK cells, polymorphonuclear leucocytes (PMNs) and fibroblasts from beige mice [14–16].
Meanwhile, ceramide has been recognized as an important second messenger in intracellular signalling [17,18]. We have reported recently that ceramide promotes calpain-mediated down-regulation of PKC in murine PMNs [19]. In leucocytes from CHS patients, sphingomyelin (SM) hydrolysis was reported to be accelerated [20]. Recently, we reported that ceramide levels which are produced by SM hydrolysis are increased in fibroblasts from beige mice compared with normal mice and that the increased ceramide causes PKC breakdown [16]. Thus we were tempted to examine whether increased ceramide is associated with the pathogenesis of CHS.
We have also demonstrated that the abnormalities of concanavalin A (Con A) cap formation and NK activity in beige mice were recovered by treatment of cells with a potent inhibitor of calpain [14,21]. In the present study, we examined whether the abnormal down-regulation of PKC activity was seen in peripheral blood mononuclear leucocytes (PBMC) from CHS patients and Epstein–Barr (EB) virus-transformed B-lymphocyte cell lines derived from CHS patients. We report here that PKC activity is abnormally down-regulated in PBMC and cell lines from CHS patients after stimulation with Con A, and that E-64-d, which is a cell-permeable calpain inhibitor [22], corrects the abnormalities in Con A cap formation and lysosomal enzyme activity by reversing the declined PKC activity.
PATIENTS AND METHODS
Materials
E-64-d was kindly provided by Dr Murata (Taisho Pharmaceutical Co.). [γ32p]ATP was purchased from Amersham Pharmacia Biotech. [3H]palmitic acid was from Moravek Biochemicals (Brea, CA, USA), and [14C]sphingomyelin was from Dupont NEN (Boston, MA, USA). Leupeptin, Z-Leu-Leu-H, chymostatin and the substrates of elastase and collagenase-like peptidase were purchased from Peptide Institute Inc. (Osaka, Japan). The substrates of cathepsin G and β-galactosidase, dipeptidyl peptidase, chelerythrine, C2-ceramide (N-acetylsphingosine), Con A, SM and other chemicals were purchased from Sigma Chemicals Co. (St Louis, MO, USA).
Patients
Peripheral blood samples were obtained from four CHS patients who had not undergone bone marrow transplantation (patient 1: 11-year-old female, patient 2: 12-year-old male, patient 3: 10-year-old female, patient 4: 7-year-old female) and two normal controls (control 1,2). Informed consent was obtained from patients or families for this study.
Cell lines
The EB virus-transformed B cell lines from three CHS patients (CHSA,B,C) and normal controls (NA,B,C) used are as follows: CHSA and NA was established by Dr Nunoi (Kumamoto University Medical School), CHSB, CHSC, NB and NC were established by Dr Agematsu (Shinshu University School of Medicine). CHSA was derived from patient 1. Cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (Filtron Pty, Australia), penicillin (100 unit/ml) and streptomycin (100 μg/ml).
Separation of peripheral blood mononuclear cells (PBMC)
To separate PBMC from CHS patients and normal controls, heparinized whole blood was centrifuged by Ficoll-Hypaque (Pharmacia Biotech AB, Uppsala, Sweden) density gradient at 400 g for 30 min. The cells were then washed twice with the medium described above and resuspended in the same medium.
Assay for PKC activity
Cells (5×106) from normal and CHS patients were cultured with or without various reagents, and were then disrupted by sonication for 20 s three times at 4°C in 20 mm Tris-HCl (pH 7·5), 0·25 m sucrose, 2 mm EDTA, 5 mm EGTA, 2 mm phenylmethylsulphonylfluoride, 0·01% leupeptin and 50 mm 2-mercaptoethanol. The cell extracts were then centrifuged at 100 000 g for 1 h at 4°C. The resultant supernatant was referred to as the cytosolic fraction. The pellets were resuspended in the same buffer containing 0·1% Triton X-100, sonicated, and centrifuged at 30 000 g for 15 min at 4 °C. The resultant supernatant was referred to as the membrane fraction. PKC activity was assayed by using PKC enzyme assay system (Amersham Pharmacia Biotech) according to the manufacturer's protocol.
Con A cap formation
Fluorescein isothiocyanate (FITC)-conjugated Con A cap formation was determined according to the method described previously [21]. After the cells (1×106/ml) were cultured in the presence or absence of E-64-d for 24 h, cells were further incubated with C2-ceramide or chelerythrine for 2 h. FITC-Con A was then added at a concentration of 20 μg/ml, and cells were incubated for 30 min at 37°C. The cells were fixed with 2% paraformaldehyde for 10 min at 37°C. Wet mounts were prepared using 10 μl of each cell mixture on a glass slide with cover slips. The cells were observed with an epifluorescence microscope fitted with an FITC interference filter and 20× or 40× objective. Scoring was carried out in two categories with respect to the distribution of the label as random clusters (diffuse of patched) or capped. Two hundred cells were counted, and the percentage of capped cells was calculated.
Assay for lysosomal enzyme activity
After the cells (5×106) from normal and CHS patients were cultured in the presence of various reagents, 1 ml of homogenate buffer (containing 20 mm imidazol-HCl (pH 7·3), 0·2% TritonX-100, 0·2 m sucrose, 5 mm EDTA) was added to the cell pellets. The cells were then disrupted by sonication for 20 s three times at 4°C and were centrifuged at 15 000 r.p.m. for 15 min at 4°C. The supernatant was used for the following enzyme assay. Elastase activity was measured with the fluorogenic substrate methoxysuccinyl-Ala-Ala-Pro-Val-4-methylcoumarinyl-7-amide(Meo-Suc-Ala-Ala-Pro-Val-MCA) by the method of Barrett [23]. The regular reaction mixture (100 μl) contained 40 mm Tris-HCl (pH 7·5), 8% (v/v) dimethylsulphoxide, 0·4 m NaCl and 0·8 mm substrate. The mixture was incubated at 37°C for 15 min after which the reaction was terminated by adding 1 ml of 5 m formic acid. Fluorescence product, AMC, was measured by its fluorescence intensity at 460 nm with excitation at 370 nm on fluorescence spectrophotometer F-4500 (Hitachi). Cathepsin G activity was assayed using Suc-Ala-Ala-Pro-Phe-PNA as substrate according to the method developed by Barrett [24]. The mixture was incubated at 50°C for 15 min after which the reaction was terminated by adding 200 μl of 0·3 mg/ml of trypsin inhibitor. The reaction product was measured at 410 nm on a DV640 Spectrophotometer (Beckman). One unit of the enzyme activity is expressed as 1 nmol products/h. The activity of β-galactosidase was assayed according to the method of Brandt et al. [25]. The activity of collagenase-like peptidase was assayed using Suc-Gly-Pro-Leu-Gly-Pro-MCA as the substrate by the method developed by Kojima et al. [26].
Sphingolipid metabolism
Cell lines (3×106) from normal and CHS patients were radiolabelled by the addition of [3H]palmitic acid (1 μCi/ml) to the culture medium for 24 h. The cells were washed with phosphate-buffered saline and further incubated with or without Con A. The medium was removed and the cells were precipitated in cold methanol (1 ml) containing 20 μg/ml each of SM and ceramide. The lipid was extracted and separated by sequential thin layer chromatography (TLC) as described previously [27]. Sphingolipid was located by iodine vapour and the silica gel was scraped and counted for radioactivity.
Assay for neutral SMase (N-SMase) and acidic SMase (A-SMase) activity
The micellar SMase assay using exogenous radiolabelled SM was performed according to the method described by Wiegmann et al. [28].
Statistics
Statistical analysis was performed with Student's t-test.
Results
Effect of E-64-d on the abnormality in Con A cap formation in CHS cell lines
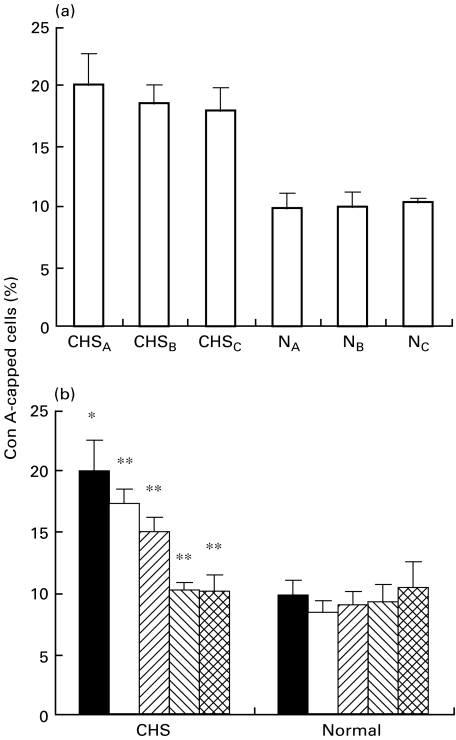
It was reported that Con A cap formation is increased in CHS cells. We first examined the Con A cap formation in three CHS cell lines. As shown in Fig. 1a, the proportion of Con A-capped cells in all CHS cell lines was apparently increased compared with that in the three control cell lines. We have demonstrated previously that abnormal down-regulation of PKC is involved in the increased Con A capping in PMNs from CHS mice [21]. We next examined the effect of E-64-d, which is a thiol proteinase inhibitor and protects PKC from its proteolysis, on Con A cap formation in CHS cell lines. E-64-d is a membrane-permeable analogue of E-64-c, and Ki of E-64-c for calpain is 0·96 μm (0·33 μg/ml) [29]. As shown in Fig. 1b, E-64-d significantly decreased the abnormally increased Con A capping in a dose-dependent manner, whereas it did not affect the capping in control cells lines. E-64-d at a concentration of 1 μg/ml corrected the capping to almost normal levels in CHS cell lines. Incubation of both cell lines with E-64-d at the concentrations used affected neither cell viability (>95% by trypan blue exclusion assay) nor cell growth (data not shown). As shown in Table 1, other protease inhibitors, leupeptin (5 μm) and Z-Leu-Leu-H (10 μm) which block calpain [14,21,30], significantly decreased the increased capping in CHS cell lines. In contrast chymostatin, which does not inhibit calpain, had no effect on Con A capping.
Fig. 1.
Effect of E-64-d on Con A cap formation in CHS and normal cell lines. (a) The cells (1×106/ml) from three CHS (CHSA,B,C) and three normal (NA,B,C) cell lines were incubated with FITC-Con A (20 μg/ml) for 30 min at 37°C, and the Con A-induced capping was determined as described in Patients and methods. (b) The cells were cultured in the presence of various concentrations of E-64-d for 24 h, and then FITC-Con A (20 μg/ml) was added and the Con A-induced capping was determined. The column represents the mean ± s.e. of at least four experiments. CHS: the mean ± s.e. of three normal cell lines. Normal: the mean ± s.e. of three normal cell lines. *P < 0·01, significant when compared with normal cell lines without E-64-d.**P < 0·01, significant when compared with CHS cell lines without E-64-d. ▪ 0; □ 0·11;  0·33;
0·33;  1;
1;  3 E‐64.9 (μg/ml).
3 E‐64.9 (μg/ml).
Table 1.
Effects of other protease inhibitors on Con A cap formation
| Cell lines | |||
|---|---|---|---|
| Inhibitors | Concentration | Normal | CHS |
| None | 10·3 ± 0·8 | 19·9 ± 1·2 | |
| E-64-d | 1 μg/ml | 9·4 ± 1·3 | 10·2 ± 0·7* |
| Leupeptin | 5 μm | 10·9 ± 1·0 | 13·2 ± 2·6* |
| Z-Leu-Leu-H | 10 μm | 10·5 ± 1·4 | 14·3 ± 1·6* |
| Chymostatin | 10 μm | 12·2 ± 2·0 | 19·6 ± 1·5 |
The cells (1 × 106/ml) from CHS and normal cell lines were cultured with indicated reagents for 24 h, and then FITC-Con A (20 μg/ml) was added and the Con A-induced capping was determined as described in Patients and methods.
P < 0·01, significant when compared with CHS cell lines without reagents.
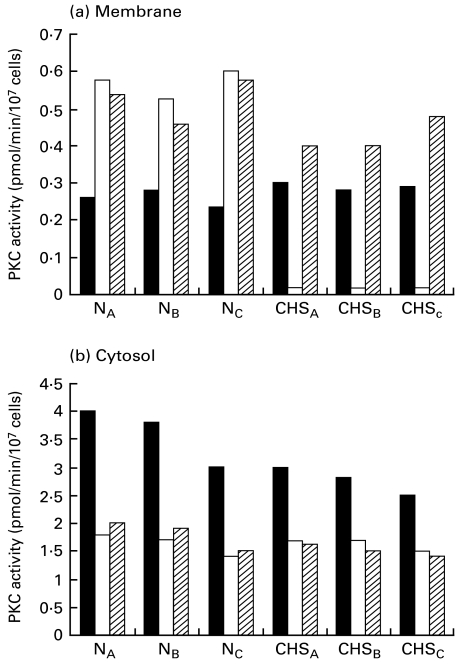
Abnormal down-regulation of PKC in Con A-stimulated CHS cell lines
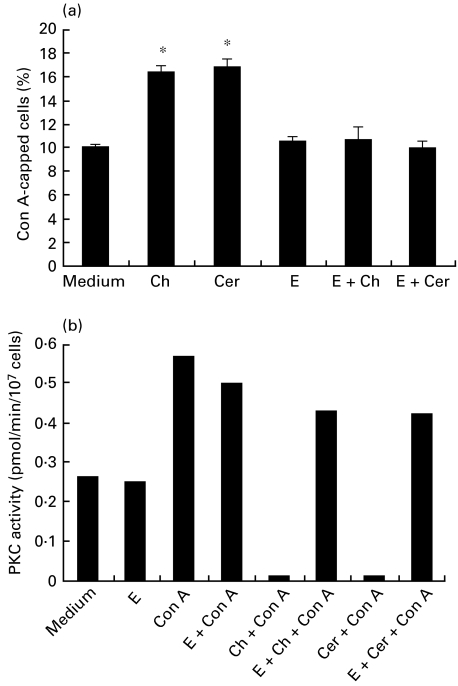
Figure 2 shows the PKC activity in CHS and control cell lines. In three control cell lines, the membrane-bound PKC activity increased after 20-min stimulation with Con A (20 μg/ml), while the cytosolic enzyme activity decreased by Con A-induced translocation. In CHS cell lines, the membrane-bound PKC activity markedly declined, while the cytosolic enzyme activity decreased by the translocation. Parallel with the decrease in the membrane-bound PKC activity in CHS cell lines, the Ca2+, phospholipid-independent kinase activity, which is the product of PKC proteolysis by calpain, increased after Con A stimulation (data not shown). When CHS cell lines were cultured overnight in the presence of E-64-d (1 μg/ml), the reduced PKC activity clearly increased (Fig. 2). E-64-d did not affect PKC activity in control cell lines. Other calpain inhibitors, leupeptin (5 μm) and Z-Leu-Leu-H (10 μm), also reversed the declined PKC activity of CHS cell lines (data not shown). We next examined whether the effect of E-64-d is associated with PKC activity. As shown in Fig. 3a, a specific PKC inhibitor, chelerythrin (1 μm), significantly increased Con A capping in control cell lines. The IC50 of chelerythin for PKC is 0·66 μm [31]. When E-64-d (1 μg/ml) was added to the culture prior to the addition of chelerythrin, both the capping and PKC reverted to normal levels (Fig. 3a,b). We also found that C2-ceramide (3 μg/ml), which has been demonstrated to promote calpain-mediated breakdown of PKC [19], significantly increased the capping in control cell lines. E-64-d also reversed both the C2-ceramide-induced increment of the capping and the declined PKC. E-64-d alone did not affect PKC activity in normal cell lines (Fig. 3b). These results indicate that the down-regulated PKC is associated with the increased Con A capping in CHS cell lines.
Fig. 2.
Effect of E-64-d on the abnormal down-regulation of PKC activity in Con A-stimulated CHS and normal cell lines. The cells (1×106/ml) from three CHS (CHSA,B,C) and three normal (NA,B,C) cell lines were cultured overnight in the presence of E-64-d (1 μg/ml) or medium alone. Then the cells were stimulated with or without Con A (20 μg/ml) for 20 min. The membrane-bound (a) and the cytosolic (b) PKC activity was assayed as described in Patients and methods. The data are means (pmol/min/107cells) of at least three experiments. The s.e. of each column was less than 10%. ▪ Medium; □ Con A;  E-64-d + Con A.
E-64-d + Con A.
Fig. 3.
Effects of various reagents on Con A cap formation and membrane-bound PKC activity in normal cell lines. (a) After the cells (1×106/ml) were cultured overnight with or without E-64-d (1 μg/ml), the cells were treated with chelerythrin (1 μm;) or C2-ceramide (3 μg/ml) for 2 h. FITC-Con A (20 μg/ml) was then added and incubated for 30 min at 37°C. Con A cap formation was determined as described in Patients and methods. The column represents the mean ± s.e. of at least four experiments. E, E-64-d; Ch, chelerythrin; Cer, C2-ceramide. *P < 0.01 compared with medium alone. (b) After the cells (1×106/ml) were treated with chelerythrin (1 μm) or C2-ceramide (3 μg/ml) for 2 h, Con A (20 μg/ml) was added and incubated for 20 min. In some experiments, cells were preincubated overnight with E-64-d (1 μg/ml) before treatment with chelerythrin or C2-ceramide. PKC activity was assayed in the membrane fractions as described in Patients and methods. The data are means (pmol/min/107 cells) of at least three experiments. The s.e. of each column was less than 10%. E, E-64-d; Ch, chelerythrin; Cer, C2-ceramide.
Abnormal PKC down-regulation and increased Con A cap formation in PBMC from CHS patients
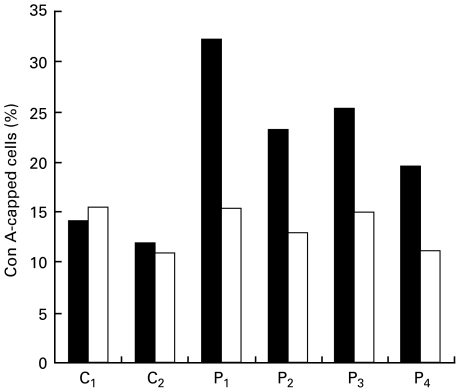
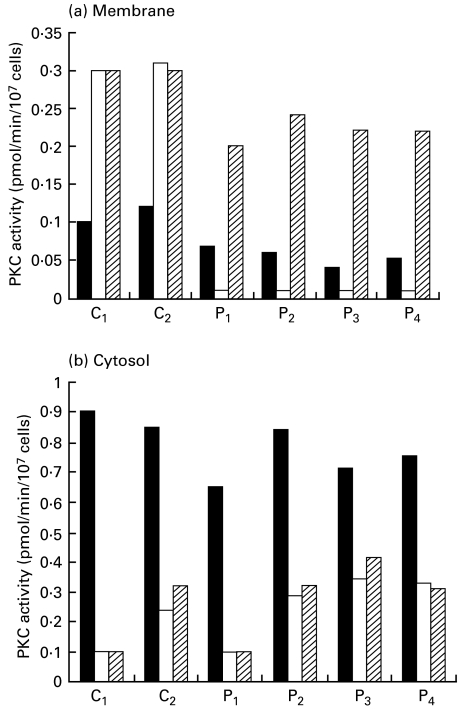
In order to clarify whether the abnormal PKC down-regulation is due to the transformation by EB virus, we examined the PKC activity and Con A cap formation in PBMC from blood samples of four CHS patients. As shown in Fig. 4, the proportion of Con A-capped cells in PBMC of all CHS patients showed a two-fold increase compared with the control. We also found that E-64-d corrected to almost normal levels. In addition, the membrane-bound PKC activity in Con A-stimulated PBMC from CHS patients declined, whereas that in normal control increased by the translocation (Fig. 5). E-64-d also reversed the decreased membrane-bound PKC activity. Incubation of PBMC with E-64-d did not alter cell viability. These results suggested that E-64-d corrected the abnormalities in PKC and Con A cap formation not only in EB virus-transformed CHS cell lines but also in PBMC from CHS patients.
Fig. 4.
Effect of E-64-d on Con A cap formation in PBMC from CHS patients and normal controls. The PBMC (1×106/ml) separated from four CHS patients (P1,2,3,4) and two normal controls (C1,2) were incubated with E-64-d (1 μg/ml) or medium alone for 1 h, and FITC-Con A (20 μg/ml) was then added and the con A-induced capping was determined as described in Patients and methods. ▪ Medium; □ E-64-d.
Fig. 5.
Effect of E-64-d on the abnormal down-regulation of PKC activity in Con A-stimulated PBMC from CHS patients and normal controls. The PBMC (1×106/ml) from four CHS (P1,2,3,4) and two normal controls (C1,2) were incubated with E-64-d (1 μg/ml) or medium alone for 1 h. Then the cells were stimulated with or without Con A (20 μg/ml) for 20 min. The membrane-bound (a) and the cytosolic (b) PKC activity was assayed as described in Patients and methods. ▪ Medium; □ Con A;  E-64-d + Con A.
E-64-d + Con A.
Effect of E-64-d on lysosomal enzyme activity in CHS cell lines
It has been reported that the lysosomal elastase and cathepsin G activity is selectively reduced in both CHS and beige cells [32,33]. We have recently reported that E-64-d almost corrects the decreased elastase and cathepsin G activity in beige fibroblasts [16]. As shown in Table 2, elastase and cathepsin G activity in CHS cell lines is significantly lowered compared with that in normal cell lines. It was found that E-64-d (1 μg/ml) significantly increased both enzymes, whereas the activity in normal cell lines was not altered by E-64-d. In contrast, chelerythrin (1 μm) significantly inhibited both elastase and cathepsin G activity. C2-ceramide, which has been demonstrated as inducing down-regulation of PKC [19], also inhibited the activity of both these enzymes. However, neither chelerythrin nor C2-ceramide altered β-galactosidase and collagenase-like peptidase> activity in normal cell lines. Incubation of cells with E-64-d for 24 h affected neither cell viability nor proliferation (data not shown). In addition, neither elastase nor cathepsin G activity was changed when E-64-d or other reagents were directly added to the enzyme assay. These findings suggest that the down-regulation of PKC may cause the reduction of elastase and cathepsin G activity, and that E-64-d may correct the reduced enzyme activity by reversing PKC activity in CHS.
Table 2.
Effects of E-64-d and other reagents on lysosomal enzyme activity in CHS and normal cell lines
| Enzyme activity | ||||||
|---|---|---|---|---|---|---|
| Cell line | Reagent | Concentration | Elastase (U/106 cells) | Cathepsin G (U/106 cells) | β-Galactosidase (%) | Collagenase- like peptidase (%) |
| Normal | Medium | 40·0±2·32 | 0·11±0.01 | 100 | 100 | |
| Chelerythrin | 1 μm | 25·2±0·80* | 0·06±0.01* | 92·9 | 93·6 | |
| C2-ceramide | 3 μg/ml | 16·0±1·92* | 0·03±0.004* | 92·8 | 89·6 | |
| E-64-d | 1 μg/ml | 38·4±1·28 | 0·09±0.008 | 99·8 | 99·5 | |
| CHS | Medium | 11·2±0·96* | 0·04±0.02* | 100 | 100 | |
| E-64-d | 1 μg/ml | 33·2±2·52† | 0·09±0.02† | 97·5 | 98·4 | |
After cells were cultured overnight in the presence of indicated reagents, the cell extracts were assayed for lysosomal enzyme activity as described in Patients and methods. One unit of elastase and cathepsin G activity is expressed as nmol products/h. Data are expressed as means ± s.e. of at least three experiments. The activity of β-galactosidase and collagenase-like peptidase is expressed as means of the percentage of the enzyme activity of each cell line cultured with medium alone. No significant difference in the activity of these enzymes was observed between normal and CHS cell lines.
P < 0·01, significant when compared with normal cell lines with medium alone.
P < 0·01, significant when compared with CHS cell lines with medium alone.
Enhanced ceramide production in Con A-stimulated CHS cell lines
We recently reported that the production of ceramide, which causes PKC breakdown, was increased in PMA-stimulated fibroblasts from beige mice [16]. Thus we examined sphingolipid levels in CHS and normal cell lines. As shown in Table 3, ceramide levels were significantly increased after Con A stimulation, whereas the levels were not altered in normal cell lines. Parallel with the increase in ceramide levels, SM levels were significantly decreased in CHS cell lines. It is considered that the increased ceramide levels in CHS cell lines were caused by the accelerated SM hydrolysis.
Table 3.
Sphingolipid levels in CHS and normal cell lines
| Sphingolipid (cpm/106 cells) | |||
|---|---|---|---|
| Cell line | Stimulation | Ceramide | Sphingomyelin |
| Normal | None | 450·4 ± 34·5 | 828·8 ± 79·6 |
| Con A | 545·7 ± 76·3 | 835·4 ± 75·7 | |
| CHS | None | 457·8 ± 21·6 | 656·1 ± 48·8 |
| Con A | 810·5 ± 44·4* | 420·6 ± 32·3* | |
After [3H] palmitic acid-labelled cells were stimulated with or without Con A (20 μg/ml) for 15 min, cellular sphingolipid was extracted and separated by TLC as described in Patients and methods. The data are means ± s.e. of three experiments.
P < 0·01, significant when compared with unstimulated CHS cells.
Enhanced SMase activity in Con A-stimulated CHS cell lines
We next examined the enzyme activity of N-SMase and A-SMase which hydrolyse SM in CHS and normal cell lines. As shown in Table 4, both N-SMase and A-SMase were significantly enhanced after Con A stimulation, whereas neither of these enzymes was changed in normal cell lines. It is known that A-SMase produces ceramide in lysosome [28]. We then examined whether Con A stimulation increased other lysosomal enzyme activity. Neither β-galactosidase activity nor collagenase-like peptidase activity was altered after Con A stimulation (Table 4). These results suggest that the increased ceramide production in CHS cell lines may be caused by enhanced SMase activity.
Table 4.
Activity of SMase in CHS and normal cell lines
| Normal | CHS | |||
|---|---|---|---|---|
| Enzymes | None | Con A | None | Con A |
| N-SMase | 248·3 ± 12·1 | 258·3 ± 17·3 | 236·2 ± 28·8 | 453·7 ± 21·0* |
| A-SMase | 693·8 ± 64·0 | 773·0 ± 15·9 | 545·5 ± 37·7 | 1028·3 ± 44·5* |
| β-galactosidase | 100 | 102·2 | 100 | 95·2 |
| Collagenase-like peptidase | 100 | 99·9 | 100 | 98·2 |
After cell lines were treated with or without Con A (20 μg/ml) for 15 min, the cells were lysed and the enzyme activity was measured as described in Patients and methods. The SMase activity is expressed as means (cpm/106 cells) ± s.e. of three experiments. The activity of β-galactosidase and collagenase-like peptidase is expressed as means of the percent of the enzyme activity of each cell line cultured without Con A.
P < 0·01, significant when compared with unstimulated CHS cells.
Discussion
We have shown here that E-64-d corrects the abnormality in Con A capping in both PBMC and B cell lines from CHS patients by reversing the down-regulated PKC activity. E-64-d is a cell-permeable, thiol proteinase inhibitor [22], and protects PKC from proteolysis by calpain. E-64-d dose-dependently decreased the abnormally increased capping in CHS cell lines at concentrations of 0·11–1 μg/ml. In addition, other calpain inhibitors such as leupeptin and Z-Leu-Leu-H, also corrected the capping in CHS. It is therefore considered that the effect of E-64-d on Con A capping is due to the inhibition of calpain. E-64-d also corrected the abnormality in lysosomal enzyme activity, which may contribute to the high susceptibility of CHS patients to bacterial infection. As we had demonstrated previously that E-64 improved not only the increased Con A capping but also the decreased NK activity in beige mice [14,21], it is possible that E-64-d is an effective drug to remedy cellular dysfunction in CHS, and to protect CHS patients from bacterial infections.
It is considered that Con A cap formation is associated with membrane–cytoskeleton interaction [34]. In PMNs from beige mice and CHS patients, it was reported that the proportion of Con A-capped cells increases compared with normal cells [35,36]. Although the increment of Con A-capped cells in CHS is associated with the defect in microtubule function, the precise mechanism is not clear. Carbachol, which increases cyclic GMP levels, had been demonstrated to recover the abnormality in the capping in CHS cells [37]. However, there is another report that the levels in cyclic nucleotide were not altered [38]. It is thus unclear whether the defect in cyclic nucleotide generation underlies this disease. We have shown previously that PKC inhibitors also enhance Con A capping in normal murine PMNs and that the abnormal rapid down-regulation of PKC occurs in Con A-stimulated PMNs from beige mice [21]. In the present report, we show for the first time that C2-ceramide, which promotes PKC breakdown, enhances Con A cap formation in human normal cell lines. It is therefore likely that PKC is tightly related to Con A cap formation.
Ceramide is known to have various effects on cellular functions such as cell differentiation, cell growth and induction of apoptosis [17,18]. Recently we reported that ceramide, which is produced by SM hydrolysis, promotes PKC breakdown induced by calpain in murine PMNs and fibroblasts [16,19]. In addition, ceramide production in beige fibroblasts was significantly increased after PMA stimulation [16]. In the present study, we have shown that ceramide production is significantly enhanced in CHS cell lines after Con A stimulation. It is known that A-SMase is associated with lysosomes. We showed that Con A stimulation increased A-SMase activity, whereas it did not alter the activity of other lysosomal enzymes (Table 4). Although we showed that both N-SMase and A-SMase were significantly enhanced after Con A stimulation in CHS cell lines, it is not clear which SMase is responsible for PKC down-regulation. In addition, it remains unknown why both SMase were enhanced.
We also showed that C2-ceramide significantly inhibits elastase and cathepsin G activity in normal cell lines, by promoting the PKC down-regulation. These two enzymes are believed to undergo similar processing in the Golgi apparatus [39]. Moreover, it was reported that elastase is present in granules as a 46-kDa proenzyme in beige neutrophils [40]. It is considered that the processing and the intracellular transport of these enzymes is deficient in CHS, and that PKC is involved in generating the active form of these enzymes by some unknown mechanism.
The genetic defect of CHS and beige mice (CHS I) has been demonstrated [5–7], however, the precise role of CHS I protein has not been elucidated. CHS I protein is 400-kDa cytosolic protein and suggested as being important in MHC class cantigen presentation [41]. This protein has a region of sequence similar to stathmin, which associates with cellular signal response [7]. Stathmin is a ubiquitous cytosolic protein which is phosphorylated on up to four sites in response to many regulatory signals, and has many phosphrylation sites for protein kinases, including PKC [7]. They suggested a relationship between the gene and the phosphorylation by PKC. However, the precise mechanism is not clear. Another functional domain is called BEACH. The BEACH domain is homologous to Vps 15, which is yeast vesicular sorting protein [6], and associates with the intracellular transport. In beige mice, it was reported that CHS I is disrupted by 5-kilobase deletion and CHS I mRNA is markedly reduced [7]. In CHS patients, the heterogenous mutations of CHS I has also been reported [8]. Further work is needed to investigate the relationship between the dysregulation of PKC and CHS I defect.
Acknowledgments
This work was supported in part by the Uehara Memorial Fund.
References
- 1.Chediak M. Nouvelle anomalie leukocytaire de caractere constitutionel et familiel. Rev Hematol. 1952;7:362–7. [PubMed] [Google Scholar]
- 2.Higashi O. Congenital gigantism of peroxidase granules. Tohoku J Exp Med. 1954;59:315–32. doi: 10.1620/tjem.59.315. [DOI] [PubMed] [Google Scholar]
- 3.Roder JC, Haliotis T, Klein M, et al. A new immunodeficiency disorder in humans involving NK cells. Nature. 1980;284:553–5. doi: 10.1038/284553a0. [DOI] [PubMed] [Google Scholar]
- 4.Jones KL, Stewart RM, Fowler M, Fukuda M, Holcombe RF. Chediak–Higashi lymphoblastoid cell lines: granule characteristics and expression of lysosome-associated membrane proteins. Clin Immunol Immunopathol. 1992;65:219–26. doi: 10.1016/0090-1229(92)90150-m. [DOI] [PubMed] [Google Scholar]
- 5.Perou CM, Moore KJ, Nagle DL, et al. Identification of the murine beige gene by YAC complementation and positional cloning. Nat Genet. 1996;13:303–8. doi: 10.1038/ng0796-303. [DOI] [PubMed] [Google Scholar]
- 6.Nagle DL, Karim MA, Woolf EA, et al. Identification and mutation analysis of the complete gene for Chediak–Higashi syndrome. Nat Genet. 1996;14:307–11. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa MDFS, Nguyen QA, Tchernev VT, et al. Identification of the homologous beige and Chediak–Higashi syndrome genes. Nature. 1996;382:262–5. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrat FJ, Auloge L, Pastural E, et al. Genetic and physical mapping of the Chediak–Higashi syndrome on chromosome 1q42–43. Am J Hum Genet. 1996;59:625–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Certain S, Barrat F, Pastural E, et al. Protein truncation test of LYST reveals heterogenous mutation in patients with Chediak–Higashi syndrome. Blood. 2000;95:979–83. [PubMed] [Google Scholar]
- 10.Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984;225:1365–70. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- 11.Kraft AS, Anderson WB. Phorbol esters increase the amount of Ca22r, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983;301:621–3. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- 12.Ito M, Tanabe F, Sato A, Ishida E, Takami Y, Shigeta S. Possible involvement of microfilaments in protein kinase C translocation. Biochem Biophys Res Commun. 1989;160:1344–9. doi: 10.1016/s0006-291x(89)80151-2. [DOI] [PubMed] [Google Scholar]
- 13.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–12. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Sato A, Tanabe F, Ishida E, Takami Y, Shigeta S. The thiol proteinase inhibitors improve the abnormal rapid down-regulation of protein kinase C and the impaired natural killer cell activity in (Chediak–Higashi syndrome) beige mouse. Biochem Biophys Res Commun. 1989;160:433–40. doi: 10.1016/0006-291x(89)92451-0. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Tanabe F, Takami Y, Sato A, Shigeta S. Rapid down-regulation of protein kinase C in (Chediak–Higashi syndrome) beige mouse by phorbol ester. Biochem Biophys Res Commun. 1988;153:648–56. doi: 10.1016/s0006-291x(88)81144-6. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe F, Cui S-H, Ito M. Abnormal down-regulation of PKC is responsible for giant granule formation in fibroblasts from CHS (beige) mice — a thiol proteinase inhibitor, E-64-d, prevents giant granule formation in beige fibroblasts. J Leukoc Biol. 2000;67:749–55. doi: 10.1002/jlb.67.5.749. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–80. [PubMed] [Google Scholar]
- 18.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–71. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 19.Tanabe F, Cui S-H, Ito M. Ceramide promotes calpain-mediated proteolysis of protein kinase Cβ in murine polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1998;242:129–33. doi: 10.1006/bbrc.1997.7934. [DOI] [PubMed] [Google Scholar]
- 20.Kanfer JN, Blume RS, Yankee RA, Wolff SM. Alteration of sphingolipid metabolism in leukocytes from patients with the Chediak–Higashi syndrome. N Engl J Med. 1968;279:410–3. doi: 10.1056/NEJM196808222790806. [DOI] [PubMed] [Google Scholar]
- 21.Sato A, Tanabe F, Ito M, Ishida E, Shigeta S. Thiol proteinase inhibitors reverse the increased protein kinase C down-regulation and concanavalin A cap formation in polymorphonuclear leukocytes from Chediak–Higashi syndrome (beige) mouse. J Leukocyte Biol. 1990;48:377–81. doi: 10.1002/jlb.48.5.377. [DOI] [PubMed] [Google Scholar]
- 22.Tamai M, Matsumoto K, Omura S, Koyama I, Ozawa Y, Hanada K. In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J Pharmacobio-Dyn. 1986;9:672–7. doi: 10.1248/bpb1978.9.672. [DOI] [PubMed] [Google Scholar]
- 23.Barret AJ. Leukocyte elastase. Meth Enzymol. 1981;80:581–8. doi: 10.1016/s0076-6879(81)80046-8. [DOI] [PubMed] [Google Scholar]
- 24.Barret AJ. Cathepsin G. Meth Enzymol. 1981;80:561–88. doi: 10.1016/s0076-6879(81)80044-4. [DOI] [PubMed] [Google Scholar]
- 25.Brandt EJ, Elliott RW, Swank RT. Defective lysosomal enzyme secretion in kidneys of Chediak–Higashi (beige) mice. J Cell Biol. 1975;67:774–88. doi: 10.1083/jcb.67.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima K, Kinoshita H, Kato T, Nagatsu T, Takada K, Sakakibara S. A new and highly sensitive fluorescence assay for collagenase-like peptidase activity. Anal Biochem. 1979;100:43–50. doi: 10.1016/0003-2697(79)90106-4. [DOI] [PubMed] [Google Scholar]
- 27.Jones MJ, Murray AW. Evidence that ceramide selectively inhibits protein kinase Cα translocation and modulates bradykinin activation of phospholipase D. J Biol Chem. 1995;270:5007–13. doi: 10.1074/jbc.270.10.5007. [DOI] [PubMed] [Google Scholar]
- 28.Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–15. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K. Reaction of calcium-activated protease (CANP) with an epoxysuccinyl derivative (E64c) and iodoacetic acid. J Biochem. 1983;93:1305–12. doi: 10.1093/oxfordjournals.jbchem.a134264. [DOI] [PubMed] [Google Scholar]
- 30.Tsubuki S, Saito Y, Tomioka M, et al. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J Biochem. 1996;119:572–6. doi: 10.1093/oxfordjournals.jbchem.a021280. [DOI] [PubMed] [Google Scholar]
- 31.Herbert J, Augerean J, Maffrand J. Chelerythrin is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–9. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 32.Vassalli JD, Granelli-Piperno A, Griscelli G, Reich E. Specific protease deficiency in polymorphonuclear leukocytes of Chediak–Higashi syndrome and beige mice. J Exp Med. 1978;22:1285–90. doi: 10.1084/jem.147.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi K, Wood H, Swank RT. Lysosomal elastase and cathepsin G in beige mice: neutrophils of beige (Chediak–Higashi) mice selectively lack lysosomal elastase and cathepsin G. J Exp Med. 1986;163:656–77. doi: 10.1084/jem.163.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourguignon LY, Bourguignon GJ. Capping and the cytoskeleton. Int Rev Cytolbo. 1984;87:195–224. doi: 10.1016/s0074-7696(08)62443-2. [DOI] [PubMed] [Google Scholar]
- 35.Oliver JM, Zurier RB, Berlin RD. Concanavalin A cap formation on polymorphonuclear leukocytes of normal and beige (Chediak–Higashi) mice. Nature. 1975;253:471–3. doi: 10.1038/253471a0. [DOI] [PubMed] [Google Scholar]
- 36.Oliver JM. Cell biology of leukocyte abnormalities — membrane and cytoskeletal function in normal and defective cells. Am J Pathol. 1978;93:221–59. [PMC free article] [PubMed] [Google Scholar]
- 37.Katz P, Roder JC, Zaytoun AM. Mechanisms of human cell-mediated cytotoxicity. II. Correction of the selective defect in natural killing in the Chediak–Higashi syndrome with inducers of intracellular cyclic GMP. J Immunol. 1982;129:297–302. [PubMed] [Google Scholar]
- 38.Boxer LA, Allen JM, Watanabe AM, Besch HR, Jr, Baehner RL. Role of microtubules in granulocyte adherence. Blood. 1978;51:1045–50. [PubMed] [Google Scholar]
- 39.Lindmark A, Gullberg U, Olsson J. Processing and intracellular transport of cathepsin G and neutrophil elastase in the leukemic myeloid cell line U937 − modulation by brefeldin A, ammonium chloride, and monensin. J Leukoc Biol. 1994;55:50–7. doi: 10.1002/jlb.55.1.50. [DOI] [PubMed] [Google Scholar]
- 40.Cavarra E, Martorana PA, Cortese S, Cambelli F, Simplicio PD, Lungarella G. Neutrophils in beige mice secrete normal amounts of cathepsin G and a 46 kD latent form of elastase that can be activated extracellularly by proteolytic activity. Biol Chem. 1997;378:417–23. doi: 10.1515/bchm.1997.378.5.417. [DOI] [PubMed] [Google Scholar]
- 41.Faigle W, Rapso G, Tenza D, et al. Deficient peptide loading and MHC class II endosomal sorting in a human genetic immunodeficiency disease: the Chediak–Higashi syndrome. J Cell Biol. 1998;141:1121–34. doi: 10.1083/jcb.141.5.1121. ee. [DOI] [PMC free article] [PubMed] [Google Scholar]