Abstract
Despite its potent antiviral activity, highly active antiretroviral therapy (HAART) only exerts a marginal effect on CD4+ T-cell regeneration in HIV-infected subjects. Combination therapies aimed at boosting T-cell activity and maturation may provide an important contribution to the restoration of immune function. Here, we report the results obtained by a two-year follow-up of a cohort of HIV-infected patients treated with a combination of HAART and interleukin-2 (IL-2). In these patients, in addition to a series of quantitative virological and immunological parameters, we investigated T-cell regeneration by an immunophenotypic assay monitoring CD4+ naïve T cells, and by analysis of thymic function, through the quantification of the excision DNA products of T-cell receptor rearrangement (TRECs) in lymphocytes. Compared with HAART alone, we found that the IL-2 combination therapy was equally effective in reducing the levels of viremia and marginally more effective in decreasing proviral DNA load. Strikingly, the IL-2 combination produced a marked increase in the number of CD4+ T cells bearing a naïve phenotype (CD45RA+, CD62L+), which was apparent for over 96 weeks after therapy. To assess whether these cells were the product of improved T-cell generation, we exploited a competitive quantitative molecular assay to quantify TRECs in peripheral blood lymphocytes. Surprisingly, we found that the levels of these molecules were unchanged in these patients. These findings indicate that improved thymic function does not account for the early rise of CD4 naïve cells in HIV-positive patients treated with IL-2, and suggest that alternative mechanisms of T-cell maturation and differentiation are responsible for this event.
Keywords: Interleukin-2, HIV, provirus, lymphocyte subsets, thymus
INTRODUCTION
Infection with HIV-1 results in a progressive destruction of immune cells and impairment of immune functions [1–4]. Highly active combination antiretroviral therapies produce sustained reduction of HIV plasma viremia and recovery of CD4+ T cells. However, recent studies suggest that these therapies are unable to eradicate viral infection and provide only a partial immune reconstitution [5–7]. Thus, alternative approaches have to be attempted in order to achieve more complete elimination of HIV and recovery of immune function. Among these novel therapeutic strategies, interleukin-2 (IL-2) has been used as a potential means to circumvent the immune impairment in HIV-positive patients [8–11].
Besides CD4 T-cell counts, reliable markers of immune reconstitution in treated HIV-positive patients are the levels of CD45RA+/CD62L+ CD4 naïve T cells and their capacity to proliferate in response to specific antigens and to increase cytokine production. We have previously shown that treatment of HIV-positive patients with IL-2 actually induces a rapid increase in CD4 cells bearing a naïve phenotype, both in peripheral blood and in lymphoid tissue, and that it increases the production of selected endogenous cytokines, such as IL-2 itself, interferon-γ and IL-16 [12–14].
As evaluated by computer-assisted tomography of the thymus, a correlation was found between the thymus size and the number of naïve T lymphocytes in peripheral blood. Therefore, the authors hypothesized that the levels of naive cells may represent a marker of thymic function [15,16]. More recently, it was shown that during intrathymic stages, T-cell precursors undergo rearrangement of the αβ T-cell receptor, resulting in the formation of T-cell receptor rearrangement excision circles (TRECs) that remain stable for a few divisions after T-cell migration from the thymus to the peripheral blood [16,17]. For this reason, the concentration of TRECs in the peripheral blood can be exploited to assess the levels of recent thymic emigrants and, accordingly, to estimate thymic function quantitatively.
In HIV infection, critical questions are whether the virus affects thymic output and whether this impairment could be reversed by current or innovative therapies [18,19]. Douek et al. showed that a substantial thymic output was maintained in healthy subjects also into late adulthood, and that HIV infection diminished TREC values in untreated patients [20]. Zhang et al. suggested that antiretroviral effective therapy was able to increase TREC levels only in those patients whose baseline TREC values were significantly lower compared with controls [21]. Finally, Hatzakis et al. found that the concentration of TRECs in the peripheral pool of T cells was moderately correlated with CD4 T-cell counts and inversely correlated with HIV-1 RNA load. These authors concluded that the concentration of TRECs complements HIV-RNA and CD4 T-cell count in predicting the rate of HIV disease progression, and possibly has a role in the pathogenesis of HIV disease [22].
The aim of this study was to investigate, in a controlled, randomized clinical trial, the effects of IL-2 and highly active antiretroviral therapy on TREC numbers in HIV-positive patients. We also examined in these patients the effects of the above-mentioned therapeutic combinations on CD4 naïve T-cell counts, HIV plasma viremia and on HIV-1 proviral load.
MATERIALS AND METHODS
Patients
The patients included in this study were enrolled to participate in a mono-institutional randomized study for the evaluation of clinical, immunological and virological effects of highly active antiretroviral therapy (HAART) plus interleukin-2 (IL-2) versus HAART alone. Patients were included if they met the following eligibility criteria: documented HIV infection, stage A1, A2, B1, B2 according to the 1993 CDC classification [23]; naïve for antiretroviral therapy or pre-treated only with the combination of two reverse transcriptase inhibitors (RTIs); CD4 counts > 200/mmc and HIV viremia > 500 RNA copies/ml in naïve patients, or two consecutive HIV viremia values 1 log above the nadir value observed during RTIs therapy in pre-treated patients; no previous IL-2 therapy; at least 18 years of age; granulocyte counts >1000/mmc; platelet counts >100 000/mmc; haemoglobin > 10 g/dl; GOT, GPT, ALF and γGT no more than three times the normal values; bilirubin 2 mg/dl or less; creatine 1·5 mg/dl or less. Thyroid abnormal function and significant cardiac, pulmonary and CNS impairment were considered as exclusion criteria.
The protocol was approved by the Institutional review board and informed consent was obtained from the participants. Patients were randomized to receive RTIs plus Indinavir, or 2RTIs plus indinavir plus IL-2. IL-2, administered s.c. with the following schedule: 6 MUI of Proleukin (Chiron, Emeryville, CA) at days 1–5 and 8–12 of a 28-day cycle for a total of six cycles (overall 24 weeks duration). IL-2 was administered once a day and just before injection, patients received 1 g of paracetamol as pre-medication. This therapeutic schedule was chosen because of the excellent immunological and clinical results obtained in previous trials where RTIs have been used in association with IL-2 [12,13]. Toxicity was evaluated according to NCI criteria as described [13]. In the case of toxicity greater than grade 2, IL-2 administration was delayed until toxicity became lower than grade 1. When toxicity of grade 2 or greater persisted for more than 2 weeks, reduction of IL-2 administration to half dosage was planned. In the case of toxicity or intolerance to Indinavir, patients were allowed to switch to Ritonavir or Saquinavir.
Eighteen healthy subjects of the same age as the HIV patients were included as controls.
CD4 and CD8 lymphocyte counts
Peripheral blood samples were obtained in EDTA and evaluated by a whole blood lysis technique [12,14]. Briefly, 100 µl of blood were added to the appropriate monoclonal antibody combination and incubated for 15 min. After incubation, samples were lysed and fixed by a commercial preparation (Immunoprep, Coulter, Milan, Italy). The four-colour CD3/CD4/CD8/CD45 monoclonal antibody combination (Coulter) was used to stain peripheral blood lymphocytes. Cell suspensions were then analysed in a Coulter EPICS XL flow cytometer.
CD4 naïve cells were measured by adding a CD4/CD45RA/CD62L monoclonal antibody combination (Dako, Milan, Italy) to 100 µl of fresh peripheral blood. Cell preparations were then lysed, fixed and analysed in a Coulter EPICS XL flow cytometer [24].
HIV plasma viremia
Plasma viremia was measured by the commercial bDNA system (Chiron Corporation, Emeryville, CA), following the manufacturer's instructions. The lower detection limit of this assay is 50 copies/ml.
HIV-1 provirus copy numbers
HIV provirus copy number in peripheral blood mononuclear cells (PBMCs) was determined by a quantitative competitive PCR approach described elsewhere [25,26]. Briefly, PBMCs were obtained from 10 ml of peripheral blood by density gradient centrifugation on Ficoll-Hypaque (Amersham Pharmacia, Little Chalfont, UK). For each patient, proviral DNA was extracted simultaneously from the different therapeutic time points (time 0, 24, 48 and 96 weeks) by standard methods [25].
Extracted DNA (2 µl) was co-amplified with decreasing amounts of the pSLPI-II competitive template in a 100 µl PCR reaction containing specific primers for the reference single copy β-globin gene (primer set PCO3–PCO4), or for the HIV-1 gene gag (primer set 1–2II). The construction and use of this plasmid for the quantification of HIV-1 nucleic acids have been described previously [25,26]. After amplification, 20 µl of each reaction were resolved in an 8% non-denaturing polyacrylamide gel, stained with ethidium bromide, visualized under ultraviolet light and analysed by densitometric scanning.
Detection of T-Cell receptor rearrangement excision circles (TRECs)
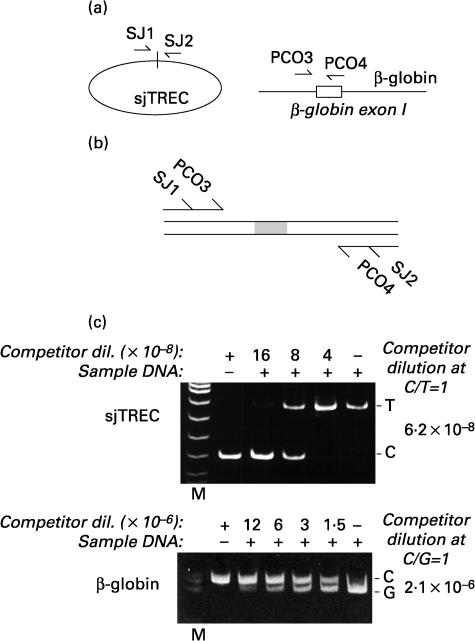
T-cell receptor rearrangement excision circles (TRECs) were detected in total PBMCs by quantitative competitive PCR. Primer sets were selected for the quantification of the β-globin gene [25], and of the extrachromosomal circle corresponding to the rearrangement event producing a signal-joint TREC [27] (Fig. 2a). For the latter amplification, primer sequences were selected located approximately 180 bp on either side of the recombination joints (molecular size of the signal joint amplification product: 360 bp). Primers for signal-joints TRECs were: 5′AAAGAGGGCAGCCC TCTCCAAGGCAAA3′ and 5′AGGCTGATCTTGTCTGACATT TGCTCCG3′.
Fig. 2.
Quantification of sjTRECs by competitive PCR. (a) Schematic representation of the localization of the two primer pairs used for quantification of sjTRECs, and of the cellular β-globin gene. (b) Competitor for competitive PCR amplification. Two long primers, corresponding to the SJ1/PCO3 and to the SJ2/PCO4 sequences, were used to amplify a DNA competitor corresponding to the β-globin PCO3-PCO4 amplification product and containing an additional 20 bp in its middle. Construction of this competitor has already been described [40]. The resulting DNA fragment contains primer recognition sequences for both the β-globin and sjTRECs primer pairs, and amplifies DNA segments of a different size to those obtained from the respective target DNA sequences. (c) Competitive PCR. For each quantification, DNA extracted from peripheral blood mononuclear cells was mixed to a different concentration of competitor (as indicated on top of each photograph) and amplified with the two primer pairs. According to the principles of competitive PCR, the ratio between amplification products for the competitor (C) and the target sjTRECS (T), or between the competitor and the target genomic DNA (G), accurately reflects the ratio of these species in the input reaction. The quantitative results obtained by the competitive PCR experiments shown in the two gels are indicated on the left side of each photograph. M: molecular weight markers.
To construct a single competitor for competitive PCR amplification, an amplification product corresponding to the β-globin segment and containing an additional 20 bp inside [25], was re-amplified with 48 nt long primers carrying, at the respective 5′ extremities, the sequences corresponding to the two strands of the sjTREC primers (Fig. 2b).
sjTREC copy numbers in PBMCs were determined by competitive PCR on genomic DNA from the mononuclear cells obtained from 10 ml of peripheral blood by density gradient centrifugation on Ficoll-Hypaque (Pharmacia). For each patient, genomic DNA was extracted simultaneously from the different therapeutic time points (time 0, 24, 48 and 96 weeks) by standard phenol–chloroform purification and ethanol precipitation procedures.
These DNAs (2 µl) were co-amplified with decreasing amounts of the competitive template in a 50 µl PCR reaction containing specific primers for the reference single copy β-globin gene (primer set PCO3-PCO4), or primers delimiting the signal joint sequence of the T-cell receptor α chain (primer set SJ1–SJ2) [27]. PCR conditions for β-globin amplification were previously reported [25]. PCR conditions for SJ primer amplification were: 95°C for 8 min, followed by 95°C and 72°C, respectively, for 30 s and 50 s, and for overall, 40 cycles. Each competitive PCR reaction contained 1·25 U Taq Gold (Perkin Elmer, Milan, Italy), 1·2 mm MgCl2, 0·2 mm dNTPs, 0·8 µm each primer. To each PCR reaction, different amounts of the diluted competitive template were added.
After amplification, 20 µl of each reaction were resolved in an 8% non-denaturing polyacrylamide gel, stained with ethidium bromide, visualized under ultraviolet light and analysed by densitometric scanning. sjTREC copy numbers were adjusted to CD4 counts and expressed taking into account the β-globin dosage, indicating the number of analysed PBMCs.
Statistical analysis
Since the variables under study were not normally distributed, non-parametric statistical tests were chosen. The Mann–Whitney test was used to compare the distributions of variables between two groups, and the Wilcoxon rank sum test was used to analyse paired values in the same group [28].
RESULTS
Here, we report the results obtained by studying the effects of the combination therapy of HAART plus IL-2, compared with HAART alone, in two cohorts of patients. Twelve patients (mean age 40·5 ± 10 years) were included in the HAART/IL-2 group; after six cycles, IL-2 was discontinued and they received HAART alone. Ten patients (mean age 39·8 ± 8 years) were included in the HAART group. The demographic, immunological and virologic characteristics of these patients at enrolment are shown in Table 1.
Table 1.
Demographic, immunological and virological characteristics of patients and controls at enrolment
| GROUP | NUM | AGE | CD4/mmc | CD8/mmc | HIV-RNA |
|---|---|---|---|---|---|
| HAART | 10 | 39 ± 8 | 320 ± 45 | 995 ± 290 | 38·700 ± 65·000 |
| HAART/IL-2 | 12 | 40 ± 10 | 300 ± 120 | 811 ± 350 | 50·700 ± 28·000 |
| Controls | 18 | 42 ± 9 | 732 ± 100 | 487 ± 133 |
Age = years; HIV-RNA is expressed as copies/ml.
All patients were observed for 96 weeks, a period that is considerably longer that other previous studies [8,9,12,13].
Viral RNA and proviral DNA load
At the time of enrolment, plasma viremia was 38 700 ± 65 000 copies/ml in the HAART group and 50·700 ± 28·000 copies/ml in the HAART/IL-2 group. In the IL-2 group, viremia was undetectable in 12/12, 11/12 and 10/12 patients at 24, 48 and 96 weeks, respectively, while in the HAART group, viremia was not detectable in 10/10 patients at 24 weeks, in 9/10 at 48 weeks and in 8/10 at 96 weeks.
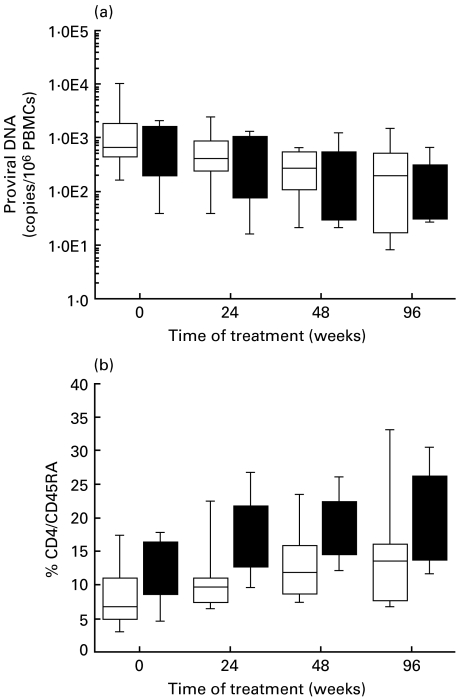
By taking advantage of a competitive PCR procedure that simultaneously measures the number of copies of HIV DNA and the cellular β-globin gene to quantify the number of mononuclear cells [25], we assessed proviral DNA levels in these patients. The results obtained (shown in Fig. 1a) indicate that both therapies produced a remarkable decline in proviral load during the first year of treatment. The mean values for pre-treatment proviral DNA levels were 2600 and 950 copies per 106 peripheral blood mononuclear cell in the HAART and HAART/IL-2 group, respectively (which were deemed not to be statistically significant), while it these were reduced to 720 and 520 copies after 24 weeks, to 320 and 370 after 48 weeks, and to 400 and 230 copies after 96 weeks, for the HAART and HAART/IL-2 group, respectively. Compared with the respective pre-treatment levels, the decrease in proviral DNA copies was significant at all time points. In both groups, however, this decrease was observed only during the first year of treatment; after this time period, the proviral DNA pool remained stable.
Fig. 1.
Proviral DNA levels and number of CD4 cells with a naïve phenotype. (a)Box plot representation of the number of proviral DNA copies in peripheral blood mononuclear cells in patients treated with HAART (□) or HAART plus IL-2 (▪) and analysed for the indicated time periods. Values were obtained by the simultaneous quantification of proviral DNA molecules and of the single copy β-globin cellular gene, a precise indicator of cell number. Quantifications were obtained using a single competitor for both measurements, as already described [25,40]. Each horizontal line in the box plot shows the 5th, 25th, 50th, 75th and 95th percentile distribution of values, from top to bottom (boxed are values from the 25th to the 75th percentiles). (b) Naïve T cells bearing the CD4/CD45RA/CD62L phenotype at different times after treatment. The box plot representation is as described in the legend for (a).
CD4 T-cell subsets
Both HAART and HAART/IL-2 combination therapies were highly active in increasing total CD4 T-cell counts at all time points, in accordance with results already reported [24]. In the HAART group, CD4 counts were 320 ± 45 cells/mmc at enrolment, 500 ± 80 cells/mmc at 24 weeks, 561 ± 195 cells/mmc at 48 weeks and 640 ± 215 cells/mmc at 96 weeks. In the IL-2 group, the values were 300 ± 120 cells/mmc before therapy, 508 ± 182 cells/mmc at 24 weeks, 585 ± 208 cells/mmc at 48 weeks and 670 ± 250 cells/mmc at 96 weeks. Compared with pre-treatment values, the increase in CD4 counts was statistically significant at all the time points in both groups. The numbers of CD4 naïve T cells bearing the CD4/CD45RA/CD62L phenotype are reported in Fig. 1b. As observed earlier [12,24], the combination therapy with HAART and IL-2 produced a significant increase in this subset of cells, starting as early as 24 weeks and persisting for the following observation periods. The mean percentage values of CD4/CD45RA/CD62L cells were 8·5% and 11·6% at enrolment, 11·5% and 18·9% after 24 weeks of therapy (P = 0·015, t = 0), 13·5% and 19·0% after 48 weeks (P = 0·037, t = 0), 15·2% and 20·5% after 96 weeks (P = 0·055, t = 0) for the HAART and HAART plus IL-2 group, respectively. CD4 naive cell values between the two groups were not statistically significant at t = 0, while they were statistically higher in the IL-2 group after 48 (P = 0·007) and 96 weeks (P = 0·05).Thus, a remarkable difference exists between the two groups, which is pronounced during therapy with IL-2 and persists for several weeks after IL-2 withdrawal.
T-cell receptor rearrangement excision circles (TRECs)
In order to understand whether the observed selective increase of CD4 T lymphocytes bearing a naïve phenotype in patients receiving IL-2 could be correlated with increased thymic output, we quantitatively determined the levels of signal joint T-cell receptor rearrangement excision circles (sjTRECs) in the two groups of patients. Previously reported evidence indicates that measurement of TRECs is a valuable indicator of lymphocytes which recently underwent T-cell receptor rearrangement, and a reliable marker of thymic function [17,20–22]. To determine the levels of TRECs quantitatively, we developed a quantitative competitive PCR procedure that measures the number of sjTRECs and the number β-globin DNA molecules in the same DNA samples. This procedures takes advantage of a single competitor DNA fragment which contains primer recognition sequences for both amplification species and is added to the DNA samples to be quantified. This strategy allows for standardization of sjTREC measurement in total extracted DNA, and takes into account variations in DNA extraction and amplification efficiency that occur among different samples. A schematic outline of this competitor and the results of a representative competitive PCR experiment are shown in Fig. 2b and 2c, respectively.
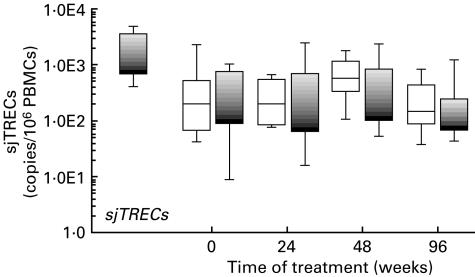
The levels of sjTREC DNA molecules in peripheral blood mononuclear cells in the patients treated with HAART or HAART/IL-2 are presented in the box plots of Fig. 3, which show the percentile distributions of the values for the two groups at the various time points. At study entry, the mean sjTREC levels were 600 molecules per 106 peripheral blood mononuclear cells in the HAART group, and 370 molecules in the HAART/IL-2 group. In agreement with other studies that measured sjTREC levels in patients with HIV infection [20,21], these values were significantly lower than those detected in a group of normal uninfected individuals (mean 1990 sjTRECs molecules/106 PBMCs). During treatment, the mean levels of sjTRECs in both groups of patients did not show remarkable differences as compared with pre-treatment levels (300, 760 and 400 molecules/106 PBMCs in the HAART group, and 650, 630 and 230 molecules/106 PBMCs in the HAART/IL-2 groups at 24, 48 and 96 weeks, respectively).
Fig. 3.
Results of quantification of sjTRECs in patients treated with HAART (□) and HAART plus IL-2 (▪) at different time points during therapy. The box plot representation is as described in the legend of Fig. 1a. Quantification of sjTRECs in a group of normal individuals is shown in black (▪). The levels of sjTRECs are expressed as number of DNA molecules per peripheral blood mononuclear cell, according to the simultaneous quantification of sjTRECs and β-globin cellular gene copy numbers.
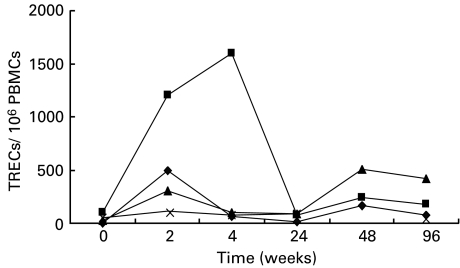
We also analysed individual TREC variations in patients with the lowest level of TREC before therapy. Figure 4 shows that injection of IL-2 produced an early and transient increase of TREC levels in these patients.
Fig. 4.
Longitudinal analysis of TRECs in patients with very low pre-treatment TREC values. Individual TREC values showed a distinct pattern of variations in patients treated with HAART and IL-2 versus HAART alone. (♦), Patient no. 8, HAART + IL-2; (▪), patient no. 11, HAART + IL-2; (▴), patient no. 8, HAART; (×), patient no. 10, HAART.
These results indicate that neither HAART nor HAART in combination with IL-2 had a long-term impact on the extent of thymic lymphocyte regeneration in these patients. However, in two patients with very low TREC levels before therapy, the early rise of TREC levels observed during IL-2 injections may deserve a more detailed investigation in clinical trials involving a larger number of IL-2-treated patients.
DISCUSSION
Infection with HIV-1 clinically correlates with progressive reduction of the CD4 T-lymphocytes subset in peripheral blood and lymphoid organs. In the last few years, the mechanisms by which HIV depletes the lymphoid compartment have been a subject of several, often contrasting, studies [1,2,29,30]. Recently, Hellerstein and co-workers provided compelling evidence that the decrease of CD4 T cells in HIV infection is caused by shortened survival time and failure to increase T-cell production [29]. One potential site of CD4 T-cell renewal blockade could be the thymus and therefore, much interest has been generated about the possibility of finding therapeutic options able to increase thymic T-cell output. Eventually, these therapeutic modalities could result in a potentiation of the immune reconstitution in HIV-infected patients. Initial studies by Zhang et al. on the effects of antiretroviral therapy on thymic functions suggested that HAART had no appreciable effects on the extent of thymic regeneration in individuals whose TRECs baseline values were within the normal range. In contrast, significant increases were observed in those with a pre-existing impairment [21]. In a very recent study, Hazemberg et al. [31] did not find any TRECs increase in HIV patients after one year of antiretroviral therapy. These authors suggested that a rapid decrease in T-cell division during therapy resulted in a slow recovery of TRECs content. In this study, we have explored the relationship between thymic function and response to IL-2 associated with antiretroviral therapy in a group of HIV-positive patients, treated with a combination of HAART and IL-2, who were analysed for almost two years after initiation of therapy. From a virological standpoint, we observed that both therapy with HAART alone and HAART plus IL-2 produced a remarkable reduction in plasma viremia and a consensual decline of proviral load in peripheral blood during the first year of treatment. After this period, even in patients with persistent suppression of plasma viremia, the proviral pool did not undergo appreciable variations. These findings reinforce the notion that HAART alone fails to eradicate HIV infection. They also indicate that the additional use of IL-2 seems to have a limited extra benefit in purging cells constituting the latent HIV reservoir. This conclusion is in apparent contrast to our previous findings, obtained by analysing the combination of IL-2 with less potent antiretroviral combinations [26], where a remarkable additive effect of the cytokine was observed early on, on the proviral DNA pool of treated patients. The findings also disagree with those of other groups [32–34] who observed a substantial reduction in the pool of resting CD4+ T cells that contain replication-competent HIV-1 in patients treated with this cytokine. In our patients, it might be envisaged that the marginal effects of IL-2 on HIV DNA purging could be related to the powerful activities of HAART during the first year of treatment, and that the additional effect of the cytokine could require longer observation periods to be appreciated.
In contrast to the above-mentioned virological findings, IL-2 produced more remarkable effects on immunological parameters. We have already observed that treatment of HIV patients with this cytokine produces an expansion of selected CD4 and CD8 sub-populations, promotes the functional recovery of CD4 subsets, and influences the production of cytokines by various cell types [12,14,24]. In particular, we confirm here that in patients treated with IL-2, a remarkable expansion of CD4 T cells bearing the naïve CD45 RA/CD62L phenotype occurs, and that this expansion lasts for as long as several months after discontinuation of IL-2 administration. Surprisingly, analysis of the sjTREC levels revealed that thymic function is not increased in patients with early stage HIV disease, thus suggesting that the expanded naïve CD4 T cells are not recent thymic emigrants. Our limited observation concerning the increase in TREC levels in patients with the lowest numbers of recent thyimic emigrants before therapy, and the recent data from Saint-Mezard et al. in patients with CD4 < 200/mmc, may suggest a role for IL-2 of increasing thymic function in those subjects with advanced immunosuppression [35]. It could be argued that an elevated T-cell division rate, which may occur during IL-2 therapy, could obscure the interpretation of TRECs data [36]. As far as this is concerned, unpublished evidence recently obtained by measuring Ki67 expression in CD4 and CD8 subsets suggests that increased T-lymphocyte proliferation could affect TRECs content only during the first two to four weeks of IL-2 administration, while it is no longer observed for more extended periods of treatment (Caggiari et al. submitted). Although a dilution effect of IL-2 treatment on TRECs measurement cannot be ruled out, it is probably limited to the first month of treatment, while later on, the results obtained in the IL-2 group and in the HAART group of patients do not reflect different kinetics of proliferation. This observation indicates that most probably, an increase in thymic function does not account for the rise in the levels of naïve T cells in IL-2-treated patients. Recently, Sempowski et al. [37] studied the effect of thymectomy on peripheral blood T cells in myasthenia gravis. These authors concluded that thymectomy resulted in a decrease in TREC levels, while no significant effect on naive T-cell numbers was observed. This observation reinforces the possibility, already suggested by other in vitro and in vivo results [38,39], that naïve T cells can derive either from memory cells reverting to a naïve phenotype after immunological activation by an appropriate stimulus such as antigenic challenge or IL-2 itself, or from extra-thymic sites. In conclusion, although several studies have investigated the immune system in HIV disease, the mechanisms contributing to the immune recovery in HIV-positive-treated patients still remain a matter for discussion and further investigation.
Acknowledgments
This work was supported by Grants from the II and III AIDS Projects of the Istituto Superiore di Sanità, Rome, Italy to P.D.P. and to M.G.
REFERENCES
- 1.Pantaleo G, Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 2.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 3.Hellerstein MK, McCune JM. T cell turnover in HIV-1 disease. Immunity. 1997;7:583–9. doi: 10.1016/s1074-7613(00)80379-9. [DOI] [PubMed] [Google Scholar]
- 4.Pantaleo G. Unraveling the strands of HIV's web. Nat Med. 1999;5:27–8. doi: 10.1038/4706. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan V, Bosche M, Metcalf JA, Ward DJ, Lane HC, Kovacs JA. HIV-1 replication in patients with undetectable plasma virus receiving HAART. Highly active antiretroviral therapy. Lancet. 1999;353:119–20. doi: 10.1016/s0140-6736(05)76156-0. [DOI] [PubMed] [Google Scholar]
- 6.Furtado MR, Callaway DS, Phair JP, et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340:1614–22. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 7.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs JA, Baseler M, Dewar RJ, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. N Engl J Med. 1995;332:567–75. doi: 10.1056/NEJM199503023320904. [DOI] [PubMed] [Google Scholar]
- 9.Davey RT, Jr, Chaitt DG, Albert JM, et al. A randomized trial of high- versus low-dose subcutaneous interleukin-2 outpatient therapy for early human immunodeficiency virus type 1 infection. J Infect Dis. 1999;179:849–58. doi: 10.1086/314678. [DOI] [PubMed] [Google Scholar]
- 10.Gea Banacloche JC, Lane HC. Immune reconstitution in HIV infection. AIDS. 1999;13(Suppl.):S25–S38. [PubMed] [Google Scholar]
- 11.Emery S, Lane HC. Immune reconstitution in HIV infection. Curr Opin Immunol. 1997;9:568–72. doi: 10.1016/s0952-7915(97)80112-4. [DOI] [PubMed] [Google Scholar]
- 12.De Paoli P, Zanussi S, Simonelli C, et al. Effects of subcutaneous interleukin-2 therapy on CD4+ subsets and in vitro cytokine production in HIV+ subjects. J Clin Invest. 1997;100:2737–43. doi: 10.1172/JCI119819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonelli C, Zanussi S, Sandri S, et al. Concomitant therapy with subcutaneous interleukin-2 and zidovudine plus didanosine in patients with early stage HIV infection. J Acquir Immune Defic Syndrome Hum Retrovirol. 1999;20:20–7. doi: 10.1097/00042560-199901010-00003. [DOI] [PubMed] [Google Scholar]
- 14.De Paoli P, Zanussi S, Caggiari L, et al. Kinetics of lymphokine production in HIV+ patients treated with highly active antiretroviral therapy and interleukin 2. J Clin Immunol. 1999;19:317–25. doi: 10.1023/a:1020547826191. [DOI] [PubMed] [Google Scholar]
- 15.McCune JM, Loftus R, Schmidt DK, et al. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest. 1998;101:2301–8. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong F, Chen CH, Cooper MD. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- 17.Poulin JF, Viswanathan MN, Harris JM, et al. Direct evidence for thymic function in adult humans. J Exp Med. 1999;190:479–86. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dybul M, Kinter A, Ruiz M, Fauci AS. Promethean thymus? J Clin Invest. 1999;101:2299–300. doi: 10.1172/JCI3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes BF, Hale LP, Weinhold KJ, et al. Analysis of the adult thymus in reconstitution of T lymphocytes in HIV-1 infection. J Clin Invest. 1999;103:453–60. doi: 10.1172/JCI5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Lewin SR, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999;190:725–32. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatzakis A, Touloumi G, Karanicolas R, et al. Effect of recent thymic emigrants on progression of HIV-1 disease. Lancet. 2000;355:599–604. doi: 10.1016/S0140-6736(99)10311-8. [DOI] [PubMed] [Google Scholar]
- 23.Center for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 24.Zanussi S, Simonelli C, Bortolin MT, et al. Immunological changes in peripheral blood and in lymphoid tissue after treatment of HIV-infected subjects with highly active anti-retroviral therapy (HAART) or HAART + IL-2. Clin Exp Immunol. 1999;116:486–92. doi: 10.1046/j.1365-2249.1999.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comar M, Simonelli C, Zanussi S, et al. Dynamics of HIV-1 mRNA expression in patients with non-progressive HIV-1 infection. J Clin Invest. 1997;100:893–903. doi: 10.1172/JCI119605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanussi S, Simonelli C, Bortolin MT, et al. Dynamics of provirus load and lymphocyte subsets after IL-2 treatment of HIV-infected patients. AIDS Res Hum Retroviruses. 1999;15:97–103. doi: 10.1089/088922299311529. [DOI] [PubMed] [Google Scholar]
- 27.Verschuren MC, Wolvers-Tettero IL, Breit TM, Noordzij J, van Wering ER, van Dongen JJ. Preferential rearrangements of the T cell receptor-delta-deleting elements in human T cells. J Immunol. 1997;158:1208–16. [PubMed] [Google Scholar]
- 28.Armitage P, Berry G. Statistical methods in medical research. Oxford: Blackwell Scientific Publications; 1987. [Google Scholar]
- 29.Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–9. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 30.Wolthers KC, Schuitemaker H, Miedema F. Rapid CD4+ T-cell turnover in HIV-1 infection: a paradigm revisited. Immunol Today. 1998;19:44–8. doi: 10.1016/s0167-5699(97)01188-2. [DOI] [PubMed] [Google Scholar]
- 31.Hazemberg MD, Otto SA, Cohen-Stuart JW. Naïve T cell division affects the TREC content of naïve T cell population. No evidence for thymic dysfunction in HIV infection; Program and Abstracts of the 7th Conference on Retroviruses and Opportunistic Infections San Francisco; January 30–February 2, 1999, abstract125. [Google Scholar]
- 32.Cooper DA, Emery S. Latent reservoirs of HIV infection: flushing with IL-2? Nat Med. 1999;5:611–2. doi: 10.1038/9454. [DOI] [PubMed] [Google Scholar]
- 33.Williams N. Can IL-2 smoke out HIV reservoirs? Science. 1998;282:1394–5. doi: 10.1126/science.282.5393.1394b. [DOI] [PubMed] [Google Scholar]
- 34.Chun TW, Engel D, Mizell SB, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–5. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 35.Saint-Mezard P, Tubiana R, De Sa M, et al. Increase of thymic production (TRECs) after adjuvant scIL2 therapy in advanced patients treated by HAART; 8th Conference on Retroviruses and Opportunistic infections, Chicago; February 4–8, 2001, abstract350. [Google Scholar]
- 36.Hazemberg MD, Otto S, Cohen-Stuart J, et al. Increased cell division but not thymic dysfunction rapidly affects TRECs content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 37.Sempowski GD, Thomasch JR, Gooding ME. Effect of thymectomy on human peripheral blood T cell pools in myasthenia gravis. J Immunol. 2001;166:2808–17. doi: 10.4049/jimmunol.166.4.2808. [DOI] [PubMed] [Google Scholar]
- 38.Bell EB, Sparshott SM. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990;348:163–6. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 39.Walker RE, Carter C, Muul L, et al. Peripheral expansion or pre-existing mature T cells is an important means of CD4+ T-cell regeneration HIV-infected adults. Nat Med. 1998;4:852–6. doi: 10.1038/nm0798-852. [DOI] [PubMed] [Google Scholar]
- 40.Comar M, Marzio G, D'Agaro P, Giacca M. Quantitative dynamics of HIV-1 expression. AIDS Res Hum Retrov. 1996;12:117–26. doi: 10.1089/aid.1996.12.117. [DOI] [PubMed] [Google Scholar]