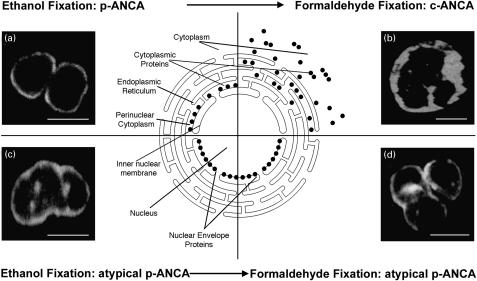
Fig. 3.
Microscopic immunofluorescence patterns of p-ANCA compared to atypical p-ANCA in relationship to the neutrophil fixation. Antineutrophil antibodies bound to their target proteins were detected by FITC-conjugated goat antihuman IgG secondary antibodies. To visualize the different fluorescence patterns, confocal laser scanning microscopy was used. The schematic diagram shows the subcellular distribution of the target antigen (•) of p-ANCA and atypical p-ANCA with respect to the used fixative agent. (a) On ethanol-fixed neutrophils, p-ANCA gave a fine perinuclear fluorescence labelling with the responsible target proteins collapsing in the perinuclear cytoplasm. (b) Using the cross-linking fixative formaldehyde, p-ANCA diffusely labelled the cytoplasm of ethanol-fixed neutrophils. A highlighting of the fluorescence between the nuclear lobes as frequently seen with c-ANCA on ethanol-fixed neutrophils was not observed. As depicted in the schematic diagram, the target proteins are diffusely distributed throughout the cytoplasm when formaldedhyde is used as fixative. (c) On ethanol-fixed neutrophils, atypical p-ANCA react with neutrophil-specific proteins in the nuclear periphery of neutrophils and produce a broad rim-like fluorescence pattern along with multiple intranuclear fluorescent foci. (d) The localization of the nuclear antigen recognized by atypical p-ANCA is not affected by the formaldehyde fixation. The staining pattern produced by atypical p-ANCA on formaldehyde-fixed neutrophils is identical to that on ethanol-fixed neutrophils. Size bars in the micrographs indicate 10 µm.