Fig. 5.
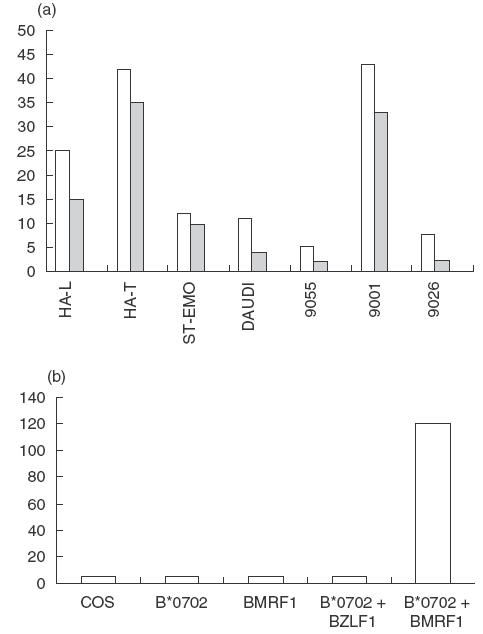
Anti-EBV T-cell response. (a) An anti-EBV T-cell line was raised by stimulating PBMCs from patient HAL with autologous EBV-B cells. The T cells were tested for cytotoxicity towards autologous EBV-B cells (HA-L, TAP2–/TAP2–, A*03011, –B*07021, –Cw0702), hemizygous cells (HA-T, TAP2+/TAP2–), ST-EMO cells (TAP2–/TAP2–, HLA-A*0301), and the TAP2+ cell lines IHW 9055 (HLA-A*0301), IHW 9001 (HLA-B*0702, HLA-Cw*0701), IHW 9026 (HLA-mismatched) and Daudi (β2m-deficient). (□) 10/1; ( ) 3/1; (▪) 0/1. (b) Antigen specificity of an anti-EBV T-cell clone. COS cells co-transfected with HLA-B*0702 and BMRF1 cDNAs were incubated for 48 h with T cells. Controls included COS cells transfected with HLA-B*0702 cDNA alone, or BMRF1 cDNA alone, or HLA-B*0702 and BZLF1 cDNA, an irrelevant EBV protein. The TNFα release (ng/ml) of the T cells was assessed by its cytotoxic effect on WEHI cells after 18h.
) 3/1; (▪) 0/1. (b) Antigen specificity of an anti-EBV T-cell clone. COS cells co-transfected with HLA-B*0702 and BMRF1 cDNAs were incubated for 48 h with T cells. Controls included COS cells transfected with HLA-B*0702 cDNA alone, or BMRF1 cDNA alone, or HLA-B*0702 and BZLF1 cDNA, an irrelevant EBV protein. The TNFα release (ng/ml) of the T cells was assessed by its cytotoxic effect on WEHI cells after 18h.
