Abstract
To investigate the changes of CD28 and HLA-DR molecules on CD4+ and CD8+ T cells during HIV infection, we classified 130 HIV-infected Koreans into four groups by the CD4 level as follows: group I (≥500 cells/mm3), group II (201–499 cells/mm3), group III (51–200 cells/mm3), and group IV (≤50 cells/mm3). In CD4+ T cells, the proportion of CD28 expression decreased significantly with the CD4 level while the proportion of HLA-DR expression increased gradually. In particular, the changes of HLA-DR expressions on CD4+ T cells were parallel to the loss of CD28 molecules from stage III to IV. However, the CD28 expression on CD8+ T cells decreased dramatically in the early stage of HIV infection, and the sum and pattern of CD28 and HLA-DR expressions on CD8+ T cells was stable after the first stage. Even though CD28 down-regulation on CD8+ T cells was very severe from the early stage of HIV infection, it might not influence the survival time of HIV-infected Koreans. The sum of the CD28+ subsets and HLA-DR subsets in each T cell was stable in all stages of disease progression. The sums of the CD28+ subsets and HLA-DR+ subsets in CD4+ T and CD8+ T cells were constant as approximately 100% and 55–60% of each T cell. These results suggested that the changes of CD28/HLA-DR expressions on CD4+ T cells were more predictable than those on CD8+ T cells in the evaluation of the disease progression during HIV-infected periods. However, we need further studies to understand why the sum of two molecules in each T cell are constant.
Keywords: HIV, CD4 level, HLA-DR molecule, CD28 molecule, disease progression
INTRODUCTION
Highly active antiretroviral therapy (HAART) has been shown to produce a dramatic decrease of HIV RNA level in plasma and lymphoid tissue [1]. These therapies have been associated with a reduced incidence of opportunistic infections and with lower mortality [2,3]. Several other studies reported that antiretroviral treatment could correct the immunological abnormalities observed in CD4+ and CD8+ T cells at the time of primary HIV infection [4–6]. Nevertheless, in most cases, the complete immune reconstitution during HAART has not been achieved [7], and controversies over the recommendation to start antiretroviral treatment in the early stages of HIV-1 infection still exist. Recently, the recovery of lymphocyte functions in HIV-infected persons has been used as an indicator of effective anti-HIV therapies [8]. Because HIV-1 RNA levels become less important as a predictor of disease progression during asymptomatic periods [9], the monitoring of CD28 and HLA-DR expressions on CD4+ and CD8+ T cells is required to manage and make the decisions for the treatment of asymptomatic HIV-infected patients [10].
The CD28 molecule, a receptor for co-stimulatory signals provided by the B7 molecule on antigen-presenting cells (APCs), is the best known second signal in human T lymphocyte activation via the T-cell receptor complex (TCR). The CD28 molecule is expressed on >95% of CD4+ T cells and approximately 50% of CD8+ T cells among peripheral blood lymphocytes (PBL) in normal people [11,12]. Engaging the T-cell receptor in the absence of CD28 co-stimulation can lead to anergy and to cell death via apoptosis in HIV-infected patients [13]. Many studies [14–17] have suggested that HIV induces dysfunction of CD4+ T and CD8+ T cells by CD28 down-regulation and by expanding CD8+ CD28– T cells, which are functionally defective CD8+ T cells.
As HLA-DR molecules are presented on most lymphoid progenitors and on activated T lymphocytes, their overexpression on T cells can reflect overactivation or immaturity [18]. Several papers have reported that HLA-DR expressions in CD4+ and CD8+ T cells of HIV-infected persons increase as their diseases progress [16,18]. However, most of these studies reported the changes of CD28 and HLA-DR molecules on CD4+ T and CD8+ T cells in HIV-infected patients classified only by clinical symptoms according to the Center for Disease Control (CDC) and/or World Health Organization (WHO) staging system.
Recent studies have reported that some HIV-infected patients have remarkable stability and have remained asymptomatic with CD4+ T-cell counts in the normal range for more than seven to 10 years following viral infection, and researchers have named them long-term non-progressors (LTNPs) [19–24]. However, studies designed only by clinical symptoms could not produce enough information about host immunological changes during the asymptomatic period because HIV-infected patients have different asymptomatic periods due to variable virological and host immunogenetical factors [25–29]. Therefore, in order to investigate the disease progression of asymptomatic HIV-infected patients, it is important to analyse the CD28 molecules as one of the co-stimulatory factors, and HLA-DR molecules as activation markers on the T subsets in HIV-infected patients classified by CD4+ T cell count.
In this study, we classified HIV-infected people into four groups according to the CD4+ T-cell categories of the 1993 revised classification of the Centre for Disease Control and Prevention (CDC) [30] and the CD4 count of ≤50 cells/mm3[31]. We analysed the correlation between the trend of CD28/HLA-DR expression on T subsets and the disease progression in HIV-infected patients. We examined which T subsets in the CD28/HLA-DR expressions show greater prognostic value for predicting disease progression during the asymptomatic periods.
PATIENTS AND METHODS
Study population
We collected blood samples from 12 normal people (all male) and 130 HIV-infected people (106 males and 24 females) for the cross-sectional study. The mean age of the 130 HIV-infected people was 36·8 ± 10·4 years (range 20–71 years). The transmission route of the 130 HIV-infected people was heterosexual contact in 91 cases, homosexual contact in 36 cases and blood transfusion in three cases. The 130 HIV-infected people were classified into four groups as follows: group I (≥500 cells/mm3), group II (201–499 cells/mm3), group III (51–200 cells/mm3) and group IV (≤50 cells/mm3). The 36 HIV-infected people belonged to group I, and there were 56 people in group II, 31 in group III and seven in group IV. The groups were defined according to the CD4+ T-cell categories of the 1993 revised classification of the CDC [30] and CD4 count of ≤50 cells/mm3, one of the important criteria in the estimation of AIDS development [31]. The mean follow-up duration of the study population was 5·3 ± 3·2 years. Thirty-four of the 130 HIV-infected people were treated with antiretroviral therapies. Two of the 34 belonged to group I, 18 to group II, 10 to group III and four to group IV. Median duration of antiretroviral treatment was 6·5 months, 22·1 months, 44·4 months and 9·0 months from group I to IV, respectively. Ninety-two HIV-infected people involved in groups I and II did not have specific clinical symptoms, but 10 of 38 HIV-infected people with less than 200 cells/mm3 of CD4+ T cells showed AIDS symptoms such as oral candidiasis, pneumonia, diarrhoea, tuberculosis and lymphoma. We measured HIV-1 viral load in about 38 HIV-infected people belonging to groups III and IV. Clinical data of HIV-infected people who belonging to groups III and IV are presented in Table 1.
Table 1.
Clinical data of 38 HIV-infected subjects with less than 200 cells/mm3 of CD4+ T cells
| No. | Sex | Age (years) | Follow-up (years) | CD4+ T (cells/mm3) | CD8+ T (cells/mm3) | Viral load* (Log10 RNA) | Therapy | Duration of antiretroviral treatment† (years) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 44 | 11·8 | 175 | 623 | 4·1 | None | |
| 2 | M | 56 | 9·9 | 75 | 1737 | 5·8 | AZT | 5·4 |
| 3 | M | 31 | 10·3 | 174 | 520 | 4·4 | None | |
| 4 | M | 57 | 9·7 | 164 | 929 | 4·6 | None | |
| 5 | M | 39 | 10·3 | 172 | 882 | 4·9 | AZT, IDV | 6·8 |
| 6 | M | 68 | 8·7 | 93 | 368 | 4·6 | None | |
| 7 | M | 50 | 15·8 | 110 | 1900 | Neg. | AZT, 3TC, IDV | 3·5 |
| 8 | M | 24 | 9·3 | 131 | 803 | 4·6 | None | |
| 9 | M | 39 | 7·4 | 196 | 593 | 4·7 | AZT | 4·9 |
| 10 | M | 43 | 6·6 | 64 | 990 | Neg. | AZT, 3TC, IDV | 3·9 |
| 11 | M | 71 | 7·1 | 116 | 1065 | 4·0 | None | |
| 12 | M | 32 | 6·6 | 56 | 326 | 5·2 | None | |
| 13 | M | 34 | 5·8 | 117 | 1076 | 4·6 | None | |
| 14 | M | 53 | 6·3 | 147 | 1384 | 4·3 | AZT | 5·2 |
| 15 | F | 28 | 5·5 | 125 | 463 | NA | None | |
| 16 | F | 29 | 5·4 | 186 | 573 | NA | None | |
| 17 | M | 50 | 4·9 | 172 | 934 | NA | None | |
| 18 | M | 42 | 4·6 | 77 | 1575 | 5·1 | None | |
| 19 | M | 36 | 4·4 | 71 | 369 | Neg. | AZT | 2·0 |
| 20 | M | 39 | 4·5 | 85 | 703 | 6·1 | AZT, 3TC, IDV | 3·4 |
| 21 | F | 21 | 1·5 | 155 | 406 | 4·5 | None | |
| 22 | F | 63 | 1·8 | 125 | 541 | 4·3 | None | |
| 23 | M | 51 | 1·6 | 58 | 221 | 4·8 | None | |
| 24 | F | 44 | 2·3 | 128 | 802 | 3·8 | AZT, 3TC, IDV | 1·3 |
| 25 | M | 22 | 0·9 | 102 | 199 | 5·4 | None | |
| 26 | F | 53 | 3·4 | 81 | 504 | 4·7 | None | |
| 27 | M | 51 | 8·9 | 95 | 919 | 5·0 | AZT, 3TC | 0·1 |
| 28 | F | 57 | 4·1 | 181 | 1316 | 6·0 | None | |
| 29 | M | 24 | 1·8 | 130 | 1124 | NA | None | |
| 30 | F | 21 | 1·4 | 186 | 703 | 4·1 | None | |
| 31 | M | 23 | 0·9 | 195 | 841 | 4·2 | None | |
| 32 | F | 33 | 4·6 | 34 | 543 | 4·9 | None | |
| 33 | F | 28 | 5·3 | 1 | 79 | 6·2 | 3TC, Nelfinavir | 0·3 |
| 34 | M | 44 | 6·0 | 36 | 185 | 4·9 | AZT | 1·1 |
| 35 | M | 51 | 2·0 | 8 | 841 | 4·3 | AZT, 3TC, IDV | 1·9 |
| 36 | M | 40 | 4·1 | 31 | 966 | 4·2 | AZT | 0·4 |
| 37 | M | 52 | 3·3 | 43 | 497 | 4·9 | None | |
| 38 | M | 42 | 0·1 | 9 | 164 | 6·2 | None |
Viral load: Log10 HIV-1 RNA; limit of detection: 400 copies/ml, Neg. Negative, NA: Not available.
Therapy: reverse transcriptase inhibitors: AZT (Zidovudine), 3TC (Lamivudine); protease inhibitors: IDV (Indinavir), Nelfinavir.
Sum of the duration of individual’s antiretroviral treatment. Even though an individual had either mono-, combination treatment or cocktail treatment, the number represents only the sum of the duration of all treatments.
Immunophenotyping by flow cytometry
Blood was collected into three potassium ethylene diamine tetra-acetic acid (K3EDTA)-treated tubes and stained within 24 h of being drawn from healthy and HIV-infected people. For flow cytometric analysis, we used the standardized whole blood lysis method to stain the K3EDTA-treated peripheral whole blood samples. We used a FACStar flow cytometer with C30 and LYSYS software (Becton Dickinson, San Jose, CA, USA). The FACStar was calibrated using a caliBRITE bead kit (Becton Dickinson) to standardize fluorescence intensity. We followed the quality control criteria recommended by the CDC [32], and the lymphocyte purity was at least 90% within the lymphocyte gate. Live gating was used to collect 10 000 events by FSC and SSC cytogram. We used two-colour flow cytometry and stained non-permeabilized intact cells to investigate cell surface expression level of CD28 and HLA-DR on CD4+ T and CD8+ T subsets in vivo. Anti-CD4, anti-CD8, anti-CD28 and anti-HLA-DR antibodies (Coulter/Immunotech, Marseille, France) were used. The fluorescein isothiocyanate (FITC)-conjugated antibodies were anti-CD28 and anti-HLA-DR. The phycoerythrin (PE)- conjugated antibody was anti-CD4 and anti-CD8.
Measurement of HIV-1 viral load in plasma
The Amplicor HIV-1 Monitor Test kit (Roche Diagnostic System, Branchburg, USA) was used to measure HIV viral load in plasma of HIV-infected patients.
Statistical analysis
We used the Mann–Whitney non-parametric method to compare certain immunological markers, including the CD4+ T cells, CD8+ T cells and the CD4/CD8 ratio between the normal group and HIV-infected group I, or between the HIV-infected groups. The Mann–Whitney test was also used to compare CD28 and HLA-DR expressions on CD4+ T and CD8+ T cells between the normal group and HIV-infected group I, or between the HIV-infected groups. The Spearman rank correlation test was used to identify any correlation between the T-cell subpopulations and CD28 or HLA-DR expression on CD4+ T or CD8+ T cells. In the Tables, the data are presented as mean ± s.d. In the Figures, the box and whisker plot was defined in terms of percentiles, where the whisker marks denote the 10th and 90th percentile, the box marks the 25th and 75th percentile and the dot in the box represents the median value. P-values of 0·05 or less were considered significant.
RESULTS
T-cell subpopulation characterization in HIV-infected groups classified by the CD4+ T level
The lymphocytes and T-cell subsets were counted in the peripheral blood of 130 HIV-infected people, classified by CD4+ T-cell categories, and 12 healthy people (Table 2). Significant differences between group I, which included the HIV-infected individuals with CD4 counts of ≥500 cells/mm3, and the normal group were found in CD4+ and CD8+ T cells (P < 0·01). According to the disease progression from stage I to IV, a significant decline was found in the CD3 counts, CD4 counts and CD4%, and CD4/CD8 ratios, except the CD8 counts and CD8%. In particular, the absolute number and percentage of CD8+ cells was relatively stable in stages II and III, but decreased dramatically in stage IV. The CD8 counts and CD8% of stage IV were 43% and 17% of those of stage III, respectively.
Table 2.
Characterization of T-cell subpopulations between normal and four HIV-1 infected groups classified by CD4 level
| Group |
|||||
|---|---|---|---|---|---|
| Variable | Normal (n = 12) | I (n = 36) | II (n = 56) | III (n = 31) | IV (n = 7) |
| Lymphocytes percentage | 38·5 ± 9·1 | 44·5 ± 8·6 | 39·3 ± 9·1†† | 37·0 ± 10·9 | 31·8 ± 15·7 |
| Lymphocytes (/mm3) | 2864 ± 878 | 3144 ± 875 | 2190 ± 650††† | 1802 ± 1017‡ | 1147 ± 688 |
| CD3+ T cells percentage | 57·8 ± 11·8 | 66·6 ± 11·3* | 62·2 ± 10·8 | 58·2 ± 13·6 | 46·8 ± 19·9 |
| CD3+ T cells (/mm3) | 1632 ± 536 | 2070 ± 595* | 1355 ± 450††† | 1004 ± 458‡‡ | 555 ± 405§ |
| CD4+ T cells percentage | 35·0 ± 7·4 | 22·9 ± 6·1*** | 16·8 ± 4·9††† | 8·5 ± 4·1‡‡‡ | 3·0 ± 3·6§§ |
| CD4+ T cells (/mm3) | 999 ± 384 | 684 ± 167** | 347 ± 76††† | 127 ± 45 | 23 ± 17§§§ |
| CD8+ T cells percentage | 29·4 ± 5·2 | 40·2 ± 10·5*** | 42·1 ± 11·3 | 46·4 ± 13·2 | 38·7 ± 15·2 |
| CD8+ T cells (/mm3) | 849 ± 326 | 1263 ± 462** | 930 ± 391††† | 819 ± 431 | 468 ± 346§§ |
| CD4/CD8 ratio | 1·2 ± 0·4 | 0·6 ± 0·3*** | 0·4 ± 0·2†† | 0·2 ± 0·1‡‡‡ | 0·05 ± 0·06§§ |
| Log10 HIV-1 RNA (copies/ml) | ND | ND | 4·7 ± 0·6 | 5·1 ± 0·8 | |
HIV-1 infected Koreans were classified into four groups by CD4 level as follows: group I (≥500 cells/mm3), group II (201–499 cells/mm3), group III (51–200 cells/mm3), and group IV (≤50 cells/mm3). Differences between HIV-infected groups were calculated using Mann–Whitney test.
Indicates significant differences between HIV-infected group I and normal group (P < 0·05)
Indicates significant differences between HIV-infected group I and normal group (P < 0·01)
Indicates significant differences between HIV-infected group I and normal group (P < 0·001))
indicates significant differences between HIV-infected group I and II (P < 0·05)
indicates significant differences between HIV-infected group I and II (P < 0·01)
indicates significant differences between HIV-infected group I and II (P < 0·001);
indicates significant differences between HIV-infected group II and III (P < 0·05)
indicates significant differences between HIV-infected group II and III (P < 0·01)
indicates significant differences between HIV-infected group II and III (P < 0·001);
indicates significant differences between HIV-infected group III and IV (P < 0·05)
indicates significant differences between HIV-infected group III and IV (P < 0·01)
indicates significant differences between HIV-infected group III and IV (P < 0·001). Data represent mean value s.d. ND: Not done.
CD28 expressions on both CD4+ and CD8+ T cells by the CD4 level
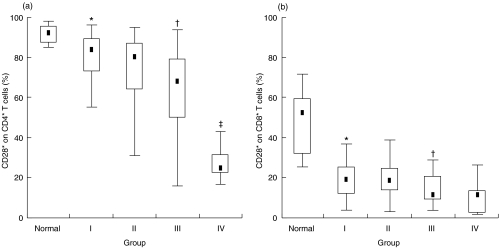
The proportion of CD28 surface molecules on each T subset, expressed as the percentage of CD4+ and CD8+ T cells, decreased dramatically in stage I. The proportion of CD28 expression on CD4+ and CD8+ T cells in HIV-infected group I was 84% and 19%, respectively. This represents a decrease of 8% and 64% from the CD28 expression values on CD4+ (92%) and CD8+ (52%) T cells, respectively, in the normal group. As shown in Fig. 1, from stage I to IV, the proportion of CD28– subsets increased gradually on CD4+ T cells, but showed relative stability on CD8+ T cells, in the HIV-infected people after the proportional CD28 expression on CD8+ T cells in stage I dropped sharply compared with that in the normal group. The CD28+/CD28– ratio on CD4+ T cells was 11:1 in the normal group but decreased dramatically to 4:1, 3:1, 2:1 and 0·4:1 by stage IV. The CD28+/CD28– ratio on CD8+ T cells was approximately 1:1 in the normal group, and there was no significant difference in all stages (0·2:1 in stage I; 0·2:1 in stage II; 0·15:1 in stage III; 0·1:1 in stage IV).
Fig 1.
CD28 expression on (a) CD4+and (b) CD8+T cells in normal and four HIV-infected groups classified by the CD4 level. The box represents the 25th and 75th percentile and the dot in the box means median value. *Significant difference between normal group and HIV-infected group I (P < 0·001); †significant difference between HIV-infected group II and III (P < 0·01); ‡significant difference between HIV-infected group III and IV (P < 0·001).
HLA-DR expressions on both CD4+ and CD8+ T cells by the CD4 level
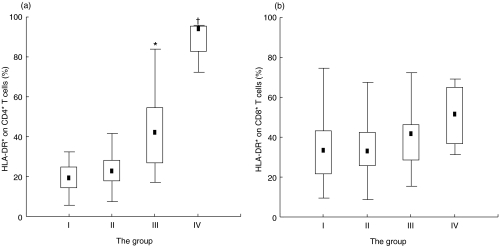
The proportion of T subsets expressing HLA-DR, one of the lymphocyte-activating markers, did not show a significant difference on CD8+ T cells, but it increased significantly on CD4+ T cells according to the disease progression from stage I to IV. In particular, as shown in Fig. 2, the proportional HLA-DR expression was two times higher than that in stage III, whereas the proportional CD28 expression was two times lower on CD4+ T cells in stage IV than on those in stage III (P < 0·01). However, the CD8+ T cells showed no significant differences in the HLA-DR and CD28 expressions during the course of stage III to IV.
Fig 2.
HLA-DR expression on (a) CD4+T and (b) CD8+T cells in four HIV-infected groups classified by the CD4 level. The box represents the 25th and 75th percentile and the dot in the box means median value. *Significant difference between HIV-infected group II and III (P < 0·001); †significant difference between HIV-infected group III and IV (P < 0·01).
Correlation between T-cell subpopulation and CD28/HLA-DR expression on each T subset
We examined the correlation between the predictive markers of HIV/AIDS and the CD28/HLA-DR expression on each T subset. As shown in Table 3, the CD28 expression on CD4+ T cells correlated with predictive markers such as CD4 counts or CD4% or the CD4/CD8 ratio (r = 0·441, P = 0·000 in CD4 counts; r = 0·500, P = 0·000 in CD4%; r = 0·566, P = 0·000 in CD4/CD8 ratio), but showed a strong negative correlation with CD8% (r = – 0·314, P < 0·001). In particular, the HLA-DR expression on each T subset correlated with the predictive markers on CD4+ T cells but not on CD8+ T cells.
Table 3.
Correlation between T-cell subpopulations and CD28 or HLA-DR expression on CD4+ and CD8+ T cells
| Variable | CD28 on CD4+T | CD28 on CD8+T | HLA-DR on CD4+T | HLA-DR on CD8+T | |
|---|---|---|---|---|---|
| Lymphocytes % | r | 0·125 | 0·003 | −0·311 | −0·333 |
| P | 0·159 | 0·978 | 0·001 | < 0·001 | |
| Lymphocytes (/mm3) | r | 0·150 | 0·072 | −0·374 | −0·144 |
| P | 0·089 | 0·416 | < 0·001 | 0·147 | |
| CD3+ T cells % | r | −0·003 | −0·265 | 0·003 | −0·031 |
| P | 0·974 | 0·002 | 0·980 | 0·755 | |
| CD3+ T cells (/mm3) | r | 0·085 | −0·057 | −0·258 | −0·149 |
| P | 0·338 | 0·522 | 0·009 | 0·132 | |
| CD4+ T cells % | r | 0·500 | 0·343 | −0·552 | −0·133 |
| P | 0·000000 | < 0·0001 | 0·000000 | 0·180 | |
| CD4+ T cells (/mm3) | r | 0·441 | 0·303 | −0·616 | −0·174 |
| P | 0·000000 | < 0·001 | 0·000000 | 0·078 | |
| CD8+ T cells % | r | −0·314 | −0·499 | 0·341 | −0·005 |
| P | < 0·001 | 0·000000 | < 0·001 | 0·958 | |
| CD8+ T cells (/mm3) | r | −0·080 | −0·217 | −0·026 | −0·084 |
| P | 0·369 | 0·013 | 0·792 | 0·401 | |
| CD4/CD8 ratio | r | 0·566 | 0·510 | −0·637 | −0·122 |
| P | 0·000000 | 0·000000 | 0·000000 | 0·220 |
Linear correlations were tested by using Spearman rank method in STATISTICA software. r: Spearman correlation coefficient.
DISCUSSION
Co-stimulatory molecules play an essential role in the optimal activation of T cells. Recent studies [15,33] showed that strong CD8+ cell antiviral responses in HIV-infected individuals were associated with a high level of CD28+ subsets. Also, several longitudinal studies showed that alterations in the CD8+ subsets could be used as a prognostic indicator for disease progression. Roos et al. [34,35] suggested that T-cell responses to the CD3 plus CD28 monoclonal antibody (MoAb) in asymptomatic HIV-infected people showed more evidence of disease progression than T-cell reactivity to CD3 MoAb alone. Landay et al. [33] reported that these changes in the levels of the CD28 and HLA-DR expressions on CD8+ cells were related to antiviral activity, and that the CD8+ CD28+ HLA-DR+ cells were responsible for natural anti-HIV activity.
Several other studies [36,37] reported that effective anti-HIV therapy induced the recovery of abnormalities in the CD8+ subsets during chronic activation, and that treatment with nucleoside analogues could correct the immunological abnormalities observed in CD4+ and CD8+ T cells at the time of primary HIV infection. However, Kanfman et al. [38] suggested that more potent drug regimens or longer-lasting therapies are required to normalize the immunological changes of CD8+ T cells during the asymptomatic periods.
We collected peripheral whole blood and stained them within at least 24 h after being drawn from healthy and HIV-infected people. The 130 HIV-infected people were divided into four groups, group I (≥500 cells/mm3), group II (201–499 cells/mm3), group III (51–200 cells/mm3) and group IV (≤50 cells/mm3), and the level of cell surface expression of CD28 and HLA-DR on CD4+ T and CD8+ T subsets in each group was measured by using two-colour flow cytometry in vivo.
In this study, through the disease progression of HIV infection from stage I to IV, the proportion of CD28 surface molecules on T subsets decreased gradually on CD4+ T cells. However, the CD28 expression on CD8+ T cells was stable after the sharp decrease of the early of HIV-infection. By switching stage III and IV, the expression of the CD28 and HLA-DR molecules showed a significant reverse correlation on CD4+ T cells, but not on CD8+ T cells. Furthermore, we investigated the influence of anti-HIV treatment and HIV-1 viral load in CD28 and HLA-DR expression on CD4+ T and CD8+ T cells. As presented in Table 1, only 14 among 38 HIV-infected persons (group III and IV) were treated with anti-HIV drugs, and the level of HIV-1 viral load did not show a significant difference between group III and IV (Table 2). Although the duration of antiretroviral treatment was slightly different from groups I to IV, the level of antiviral treatment and HIV-1 viral load in this study might not affect the patterns of CD28 and HLA-DR expression on each T subset.
As described in our previous report for the Korean long-term non-progressors and rapid progressors [39], the results of this study showed that the CD28 expressions on CD8+ T cells during all stages of HIV infection were very different from those of Brinchmann et al. [40] and Vingerhoets et al. [15]. In particular, CD28 expression on CD8+ T cells in stage I decreased significantly compared with those of HIV-infected Caucasians with similar CD8+ T counts. Several studies [41–43] have reported that CD8+ CD28– T cells accumulated when there was prior viral infection such as CMV (cytomegalovirus). Hooper et al. [43] reported that cytomegalovirus seropositivity was associated with the expansion of CD4+ CD28– and CD8+ CD28– T cells in rheumatoid arthritis. We also reported that positive rates of anti-CMV-IgG in the serum from HIV-infected and normal Koreans were 98·6% and 92·0%, respectively [44].
Therefore, if CD28 down-regulation on CD8+ T cells is influenced by a prior viral infection such as CMV and hepatitis virus, the CD28 expressions on CD8+ T cells in normal people should show a difference between Koreans and Caucasians because of the relatively high prevalence of infection of CMV and hepatitis B virus in Koreans. However, our results showed that the proportion of CD28 expressions on CD8+ T cells in normal Koreans was no different from that of Caucasians. This means that the rapid CD28 down-regulation on CD8+ T cells in HIV-infected Koreans did not correlate with the infections of other chronic viruses except HIV. If so, why do HIV-infected Koreans show a significant difference to Caucasians in the pattern of CD28 down-regulation on CD8+ T cells? One of the possible assumptions is that HIV co-infection in the status of infection of chronic viruses may accelerate CD28 down-regulation on CD8+ T cells.
The median survival time from 200 cells/mm3 of CD4+ T cells in 86 HIV-infected Koreans and from 50 cells/mm3 of CD4+ T cells in 70 AIDS patients was 3·62 years (43·4 months) and 1·65 years (19·8 months), respectively (unpublished data). These median survival times were similar to the 3·17 years (38 months) of the San Franciso Cohort study of homosexual men [45] in 200 cells/mm3 of CD4+ T cells and 1·34 years (16·1 months) of Apolonio et al. [31] in 50 cells/mm3 of CD4+ T cells. This result also shows that even though CD28 down-regulations on CD8+ T cells in Koreans are very different from those of Caucasians, they do not influence the survival time of HIV-infected Koreans.
Vingerhoets et al. [17] reported that a significant down- regulation of the CD28 expression was shown on both CD4+ and CD8+ T cells from HIV-infected people, and that CD28 down- regulation on CD8+ T cells in HIV-infected people was more clearly disease stage-related than their CD4+ T cells. On the other hand, Choremi-Papadopoulou et al. [46] showed that a positive significant correlation within the CD4+ but not the CD8+ subset was observed between the percentage of cells lacking the CD28 molecules and the percentage of cells expressing the HLA-DR and CD38 molecules. This result suggests that the loss of CD28 expression on CD4+ T cells may be associated with the functional defects of the T cells in HIV-1 infected individuals.
Our data showed that CD28 down-regulation on the T subsets in HIV-infected people increased gradually on CD4+ T cells according to the CD4+ T-cell stages. Also, the CD28+/CD28– ratios decreased dramatically on CD4+ T cells by CD4+ T-cell stage. The HIV-infected people showed a strong positive correlation between the CD28 expression on CD4+ T cells and the predictive markers (CD4 counts, CD4% and CD4/CD8 ratio). The HLA-DR expressions on each T subset correlated with the predictive markers on CD4+ T cells, but not on CD8+ T cells.
We found an interesting phenomenon in which the sum of the CD28+ subsets and HLA-DR subsets in each T subset was stable in all stages of disease progression. In CD4+ T cells, the sum of the CD28+ subsets and HLA-DR+ subsets was approximately 100%. According to the disease progression, the CD28 expressions on CD4+ T cells decreased while the HLA-DR expressions on CD4+ T cells increased, but the sum of these subsets did not change from approximately 100% of CD4+ T cells. The sum of the CD28+ subsets and HLA-DR+ subsets in CD8+ T cells was constant as 55–60% of CD8+ T cells. Choremi-Papadopoulou et al. [46] showed that the changes of HLA-DR expressions on CD4+ T cells were parallel to the loss of CD28 molecules. They suggested that this result provided evidence that the loss of CD28 molecules on CD4+ T cells might be associated with T-cell over-activation. As shown in the results of Choremi-Papadopoulou et al., our results appeared to corroborate that the changes of HLA-DR expressions on CD4+ T cells were parallel to the loss of CD28 molecules.
These results suggested that the CD28/HLA-DR expressions on CD4+ T cells have greater predictability than those on CD8+ T cells in evaluating disease progression in HIV-infected patients.
Acknowledgments
This project was supported by the grant of the project (99-I-01-04-A-008) from the Korea Institute of Science and Technology Evaluation and Planning (KISTEP).
REFERENCES
- 1.Hoen B, Dumon B, Harzic M, et al. Highly active antiretroviral treatment initiated early in the course of symptomatic primary HIV-1 infection: results of the ANRS 053 trial. J Infect Dis. 1999;180:1342–6. doi: 10.1086/315002. [DOI] [PubMed] [Google Scholar]
- 2.Dorrucci M, Suligoi B, Serraino D, Tirelli U, Rezza G. Incidence of invasive cervical cancer in a cohort of HIV-seropositive women before and after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;26:377–80. doi: 10.1097/00126334-200104010-00016. [DOI] [PubMed] [Google Scholar]
- 3.Suarez S, Baril L, Stankoff B, et al. Outcome of patients with HIV-1-related cognitive impairment on highly active antiretroviral therapy. AIDS. 2001;15:195–200. doi: 10.1097/00002030-200101260-00008. [DOI] [PubMed] [Google Scholar]
- 4.Behbahani H, Landay A, Patterson BK, et al. Normalization of immune activation in lymphoid tissue following highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25:150–6. doi: 10.1097/00042560-200010010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman GR, Zaunders JJ, Cunningham P, et al. Rapid restoration of CD4 T cell subsets in subjects receiving antiretroviral therapy during primary HIV-1 infection. AIDS. 2000;14:2643–51. doi: 10.1097/00002030-200012010-00003. 10.1097/00002030-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Rizzardi GP, Tambussi G, Bart PA, Chapuis AG, Lazzarin A, Pantaleo G. Virological and immunological responses to HAART in asymptomatic therapy-naive HIV-1-infected subjects according to CD4 cell count. AIDS. 2000;14:2257–63. doi: 10.1097/00002030-200010200-00006. 10.1097/00002030-200010200-00006. [DOI] [PubMed] [Google Scholar]
- 7.Pakker NG, Kroon ED, Roos MT, et al. Immune restoration does not invariably occur following long-term HIV-1 suppression during antiretroviral therapy. INCAS Study Group. AIDS. 1999;13:203–12. doi: 10.1097/00002030-199902040-00008. [DOI] [PubMed] [Google Scholar]
- 8.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 9.de Wolf F, Spijkerman I, Schellekens PT, et al. AIDS prognosis based on HIV-1 RNA, CD4+ T-cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS. 1997;11:1799–806. doi: 10.1097/00002030-199715000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Choremi-Papadopoulou H, Panagiotou N, Samouilidou E, et al. CD28 costimulation and CD28 expression in T lymphocyte subsets in HIV-1 infection with and without progression to AIDS. Clin Exp Immunol. 2000;119:499–506. doi: 10.1046/j.1365-2249.2000.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsten T, Thompson CB, June CH. CD28 ligation in T-cell activation. Evidence for two signal transduction pathway. Blood. 1990;75:1531–9. [PubMed] [Google Scholar]
- 12.Linsley PS, Brady W, Grosmaire L, et al. Binding of the B-cell activation antigen B7 to CD28 costimulates T-cell proliferation and interleukin 2mRNA accumulation. J Exp Med. 1991;173:759–62. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borthwick NJ, Bofill M, Gombert WM, et al. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28– T cells. AIDS. 1994;8:431–41. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Saukkonen JJ, Kornfeld H, Berman JS. Expansion of a CD8+CD28– cell population in the blood and lung of HIV-positive patients. J Acquir Immune Defic Syndr. 1993;6:1194–204. [PubMed] [Google Scholar]
- 15.Vingerhoets JH, Vanham GL, Kestens LL, et al. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+ T cells from HIV-infected subjects. Clin Exp Immunol. 1995;100:425–33. doi: 10.1111/j.1365-2249.1995.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kammerer R, Iten A, Frei PC, Burgisser P. Expansion of T cells negative for CD28 expression in HIV infection. Relation to activation markers and cell adhesion molecules, and correlation with prognostic markers. Med Micobiol Immunol. 1996;185:19–25. doi: 10.1007/s004300050010. [DOI] [PubMed] [Google Scholar]
- 17.Vingerhoets J, Kestens L, Penne G, et al. CD8+ T cells and not CD4+ T cells are hyporesponsive to CD28- and CD40L-mediated activation in HIV-infected subjects. Clin Exp Immunol. 1997;107:440–7. doi: 10.1046/j.1365-2249.1997.d01-964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kestens L, Vanham G, Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–41. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lifson AR, Buchbinder SP, Sheppard HW, et al. Long-term human immunodeficiency virus infection in asymptomatic homosexual and bisexual men with normal CD4+ lymphocyte counts: immunologic and virologic characteristics. J Infect Dis. 1991;163:959–65. doi: 10.1093/infdis/163.5.959. [DOI] [PubMed] [Google Scholar]
- 20.Sheppard HW, Lang W, Ascher MS, et al. The characterization of non-progressors: long-term HIV-1 infection with stable CD4+ T-cell levels. AIDS. 1993;7:1159–66. [PubMed] [Google Scholar]
- 21.Keet IPM, Krol A, Klein MR, et al. Characterization of long term asymptomatic infection with human immunodeficiency virus type 1 in men with normal and low CD4+ cell count. J Infect Dis. 1994;169:1236–43. doi: 10.1093/infdis/169.6.1236. [DOI] [PubMed] [Google Scholar]
- 22.Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–16. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 23.Zanussi S, Simonelli C, d'Andrea M, et al. CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin Exp Immunol. 1996;105:220–4. doi: 10.1046/j.1365-2249.1996.d01-746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrucci A, Dorrucci M, Alliegro MB, et al. How many HIV-infected individuals may be defined as long-term nonprogressors? A report from the Italian Seroconversion Study. J Acquir Immune Defic Syndr Human Retrovirology. 1997;14:243–8. doi: 10.1097/00042560-199703010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–61. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 26.Smith MW, Dean M, Carrington M, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Science. 1997;277:959–65. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 27.Winkler C, Modi W, Smith MW, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science. 1998;279:387–91. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- 28.Hendel H, Caillat-Zucman S, Lebuanec H, et al. New class I and II alleles strongly associated with opposite patterns of progression to AIDS. J Immunol. 1999;162:6942–6. [PubMed] [Google Scholar]
- 29.Carrington M, Nelson GW, Martin MP, et al. HLA and HIV-1: Heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–52. doi: 10.1126/science.283.5408.1748. 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 30.CDC. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41:RR1–17. [PubMed] [Google Scholar]
- 31.Apolonio EG, Hoover DR, He Y, et al. Prognostic factors in human immunodeficiency virus-positive patients with a CD4+ lymphocyte count <50/μl. J Infect Dis. 1995;171:829–36. doi: 10.1093/infdis/171.4.829. [DOI] [PubMed] [Google Scholar]
- 32.CDC. 1997 Revised guidelines for performing CD4+ T-cell determinations in persons infected with human immunodeficiency virus (HIV) MMWR. 1997;46:1–29. [PubMed] [Google Scholar]
- 33.Landay AL, Mackewicz CE, Levy JA. An activated CD8+ T-cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106–16. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 34.Roos MTL, Miedema F, Meinesz AAP, et al. Low T cell reactivity to combined CD3 plus CD28 stimulation is predictive for progression to AIDS: correlation with decreased CD28 expression. Clin Exp Immunol. 1996;105:409–15. doi: 10.1046/j.1365-2249.1996.d01-794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roos MTL, Prins M, Koot M, et al. Low T-cell responses to CD3 plus CD28 monoclonal antibodies are predictive of development of AIDS. AIDS. 1998;12:1745–51. doi: 10.1097/00002030-199814000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Bisset LR, Cone RW, Huber W, et al. Highly active antiretroviral therapy during early HIV infection reverses T-cell activation and maturation abnormalities. Swiss HIV Cohort Study. AIDS. 1998;12:2115–23. doi: 10.1097/00002030-199816000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Burgisser P, Hammann C, Kaufmann D, et al. Expression of CD28 and CD38 by CD8+ T lymphocytes in HIV-1 infection correlates with markers of disease severity and changes towards normalization under treatment. The Swiss HIV Cohort Study. Clin Exp Immunol. 1999;115:458–63. doi: 10.1046/j.1365-2249.1999.00818.x. 10.1046/j.1365-2249.1999.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufmann GR, Zaunders JJ, Cunningham P, Cooper DA. Phenotypic analysis of CD8+ T lymphocytes in a cohort of HIV type 1-infected patients treated with saquinavir, ritonavir, and two nucleoside analogs for 1 year, and association with plasma HIV type 1 RNA. AIDS Res Hum Retroviruses. 1999;15:963–72. doi: 10.1089/088922299310476. 10.1089/088922299310476. [DOI] [PubMed] [Google Scholar]
- 39.Choi BS, Koo BK, Go UY, et al. The altered pattern of CD28 expression on T cell subsets in HIV-infected Koreans. Korean J Immunol. 1999;21:1–8. [Google Scholar]
- 40.Brinchmann JE, Dobloug JH, Heger BH, et al. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: Functional and clinical correlations. J Infect Dis. 1994;169:730–8. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 41.Tay-Kearney ML, Enger C, Semba RD, et al. T cell subsets and cytomegalovirus retinitis in human immunodeficiency virus-infected patients. J Infect Dis. 1997;176:790–4. doi: 10.1086/517303. [DOI] [PubMed] [Google Scholar]
- 42.Hazzan M, Labalette M, Noel C, et al. Recall response to cytomegalovirus in allograft recipients: mobilization of CD57+, CD28+ cells before expansion of CD57+, CD28– cells within the CD8+ T lymphocyte compartment. Transplantation. 1997;63:693–8. doi: 10.1097/00007890-199703150-00014. [DOI] [PubMed] [Google Scholar]
- 43.Hooper M, Kallas EG, Coffin D, et al. Cytomegalovirus seropositivity is associated with the expansion of CD4+CD28– and CD8+CD28– T cells in rheumatoid arthritis. J Rheumatol. 1999;26:1452–7. [PubMed] [Google Scholar]
- 44.Kim OJ, Kim SS, Park MS, et al. p. 18. Seroprevalence of antibodies against cytomegalovirus, varicella-zoster and epstein-barr virus among HIV seropositive individuals. Korean J Infect Dis Conference Abstract.
- 45.Osmond D, Charlebois E, Lang W, et al. Change in AIDS survival time in two San Francisco Cohorts of homosexual men 1983–93. JAMA. 1994;271:1083–7. [PubMed] [Google Scholar]
- 46.Choremi-Papadopoulou H, Viglis V, Gargalianos P, et al. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J Acquir Immune Defic Syndr. 1994;7:245–53. [PubMed] [Google Scholar]