Abstract
The possible roles of CD8+ cells in the abnormal T cell-dependent B-cell activation in Graves’ disease were investigated by analysing lymphocyte subsets in peripheral blood mononuclear cells (PBMC) and their production of soluble factors and cytokines such as IL-10 in patients with Graves’ disease, Hashimoto’s thyroiditis and normal controls. The PBMC were separated into CD8+ and CD8-depleted cells by magnetic separation columns, and cultured for 7 days with or without anti-CD40 monoclonal antibodies and IL-4. The culture supernatant was assayed for sCD23 and IL-10 using EIA, and the remaining cells were analysed by flow cytometry. Stimulation with anti-CD40 antibody together with IL-4 increased sCD23 levels and the number of CD23+ cells. The latter was further augmented by depletion of CD8+ cells. This combination of B cell stimulants increased production of IL-10 by PBMC from patients with Graves’ disease. The CD40- and IL-4-activated production of IL-10 was decreased by CD8+ cell depletion. In contrast, constitutive production of IL-10 was increased after CD8+ cell depletion in a group of patients with low basal secretion levels (<35 ng/ml). It was, however, decreased in a group with higher basal production levels, but such a relationship was not found in the normal control group. Thus, T cell-dependent B-cell activation via a CD40 pathway activates CD23+ cells, leading to over-production of IL-10 and a shift of the Th1/Th2 balance to Th2 dominance, while CD8+ cells may suppress this activation to counteract the Th2 deviation in Graves’ disease.
Keywords: B cell activation, CD40, CD8, Graves’ disease, IL-4, IL-10
INTRODUCTION
The presence of antibodies against thyroid-specific antigens such as the thyrotrophin (TSH) receptor, thyroid peroxidase (TPO) and thyroglobulin, and T and B cell infiltration in thyroid tissue reflects an autoimmune-based process in Graves’ disease [1–4]. The augmented production of B cell growth factor (BCGF; IL-14) from peripheral and thyroid-infiltrating lymphocytes [5], increased numbers of CD23+ cells [6] and the secretion of sCD23 [7] may participate in B cell activation in Graves’ disease. Activated B cells present antigens and interact with T cells by means of their expression of various co-stimulatory ligands such as CD80/CD86, which binds CD28/CTLA-4 (CD152) on T cells, and CD40 binding CD40 ligand (CD40L; CD154) on T cells [8].
T cells, especially helper T (Th) cells, play pivotal roles in the immune response; these Th cells are now divided commonly into two major subtypes depending on their ability to produce specific cytokines [9]. An imbalance of Th1 and Th2 responsiveness has been demonstrated in various autoimmune diseases. Type 1 diabetes and Hashimoto’s thyroiditis represent Th1 dominant diseases [10], while Graves’ disease is considered to show Th2 or Th0 dominance. T cells specific for the TSH receptor cloned from Graves’ disease lymphoid infiltrates produced relatively little IFN-γ and possessed distinct Th0/Th2 characteristics, unlike cloned TPO-responsive cells which have Th1 characteristics [11]. An increased accumulation of transcripts for IFN-γ, IL-2, IL-4 and IL-10 has been reported in Graves’ disease thyroid glands [12]. Another report showed that the presence of IL-2, IFN-γ, and TNF-α, but the absence of IL-4 mRNA in intrathyroidal lymphocytes indicated a pattern of cytokine production that resembled most closely that of the Th1 subset. However, those investigators emphasized that the concomitant presence of IL-10 is important in stimulating intrathyroidal autoantibody production, and suggested a role for this cytokine in inhibiting cell- mediated thyroid injury in Graves’ disease [13]. We have reported previously that the number of CD8+ cells in hyperthyroid Graves’ disease was decreased, but the number of activated CD8+ cells increased after the patients were rendered euthyroid or hypothyroid by antithyroid therapy [14]. Our previous studies also showed a possible involvement of CD8+ cells in the abnormal B cell activation with evidence of an increase of B cell growth factors [5], a decrease in CD8+ cell numbers [15] and an increase of IL-10 production [16] following activation of B cells via anti-CD40 antibody and IL-4 in peripheral blood cells from patients with Graves’ disease.
The present study was therefore carried out to elucidate the role of CD8+ cells on the T cell-dependent B-cell activation seen in Graves’ disease and to clarify the influence of CD8+ cell depletion on the Th1/Th2 balance, focusing particularly on IL-10.
PATIENTS AND METHODS
Study population
We evaluated 26 patients with Graves’ disease (one man and 25 women; 24 patients had been treated with 5–60 mg of methimazole) aged 28·3 ± 2·3 years (mean ± s.e.m.), 12 patients with Hashimoto’s thyroiditis (one man and 11 women) aged 49·3 ± 4·6 years and 12 healthy subjects (one man and 11 women) aged 26·7 ± 1·0 years, who were medical staff in our university. The diagnosis of Graves’ disease was based on clinical and biochemical indicators of hyperthyroidism, including the presence of a palpable diffuse goiter, and an elevated uptake of 123I, and a homogeneous scintigram. The serum thyroid hormone concentrations in these patients with Graves’ disease ranged from 0·21 to > 8·1 ng/dL for free T4 (normal 0·68–1·8 ng/dL), 2·7–>22·2 pg/ml for free T3 (normal 2·7–5·9 pg/ml), and < 0·01–35·1 μU/ml for TSH (normal 0·4–3·5 μU/ml). The serum thyroid hormone concentrations in patients with Hashimoto’s thyroiditis had been maintained within normal range by replacement treatment with T4 in doses ranging from 0 to 100 μg/day. Serum antibody titres to the TSH receptor (TR antibody) in Graves’ patients ranged from 1·5% to 76·7% as determined by a TR antibody kit as specified by the manufacturer (Cosmic Co., Tokyo, Japan). The antibody titres to thyroglobulin (Tg) and thyroid peroxidase (TPO) ranged from < 0·3–214 U/ml and 2·7–81·0 U/ml in Graves’ patients, and 7·6–15·2 U/ml and <0·3–125 U/ml in Hashimoto’s patients, respectively, as determined by anti-Tg and anti-TPO kits as specified by the manufacturer (Cosmic Co.).
Cell separation and culture, assay of sCD23 and IL-10 and flow cytometry
Peripheral blood was obtained after informed consent from each subject. Approximately 24 ml was collected, using heparin anticoagulation. Peripheral blood mononuclear cells (PBMC) were separated through a Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient and cultured for 7 days at 37°C at 1 × 106 cells/ml in RPMI-1640 medium containing 10% fetal bovine serum (FBS) with 2 μg/ml anti-CD40 monoclonal antibody (mouse monoclonal IgM antibody to human CD40, clone 14G7, Caltag Laboratory, San Francisco, CA, USA) and human recombinant interleukin-4 (Final conc. 10 ng/ml, Upstate Biotechnology Inc., Lake Placid, NY, USA). An equal amount of medium was added instead of antibody or IL-4 to the control, unstimulated, wells. The remaining cells were adjusted to a density of 1 × 107 cells/ml in RPMI-1640 medium containing 10% FBS, and incubated for 15 min in PBS containing 0·5% BSA, 2 mm EDTA and monoclonal antibodies-labelled magnetic beads (CD8+ T cell isolation kit; Hapten-Antibody Cocktail). After washing with the same buffer, these cells were further incubated for 10 min in PBS containing 0·5% BSA, 2 mm EDTA and monoclonal antibodies-labelled magnetic beads (CD8+ T cell isolation kit; Hapten-Antibody Cocktail), followed by separation into CD8+ and CD8-depleted populations by magnetic separation columns (MACS Separation Columns, Miltenyi Biotec, Germany) according to the manufacturer’s instructions. In this procedure, 45·1 ± 4·2% of the CD8+ cells were removed. Each set of cells was cultured for 7 days at 37°C at a density of 1 × 106 cells/ml in RPMI-1640 medium containing 10% FBS with 2μg anti-CD40 monoclonal antibody and human recombinant interleukin-4 or with an equivalent volume of the same medium. The culture supernatant was assayed for sCD23 and IL-10 and using enzyme immunoassay kits (The Binding Site Ltd, Birmingham, UK; Pharmingen, San Diego, CA, USA), and the remaining cells were subjected to two-colour flow cytometry with fluorescein isothiocyanate-labelled monoclonal antibodies to CD23 (Serotec, Oxford, UK) and CD20 (Pharmingen, San Diego, CA, USA) using a FACScan (Becton Dickinson) flow cytometer. The detector gates were set for lymphocytes by using forward and side scatter parameters such that 1 × 104 cells were collected for each graph generated.
Statistical analysis
Data are presented as mean ± s.e.m. Statistical analyses were done by anova, and used the Bonferroni–Dunn post-hoc test. The Kruskal–Wallis test was used to analyse skewed distribution. A level of P < 0·05 was accepted as statistically significant.
RESULTS
Effect of the presence or absence of CD8+ cells on anti-CD40 antibody and IL-4-activated production of soluble CD23 and CD23+ or CD20+ cells (Figs 1 and 2)
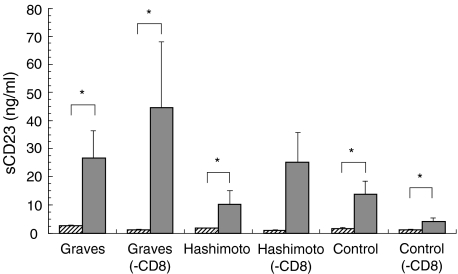
Fig. 1.
The production of soluble CD23 by PBMC or CD8-depleted cells (– CD8) from individuals with Graves’ disease or Hashimoto’s thyroiditis, and normal controls. The PBMC or CD8-depleted cells were incubated in the presence or absence of anti-CD40 MoAb and IL-4. *P < 0·05.  , (−);
, (−);  , anti-CD40 antibody and IL-4.
, anti-CD40 antibody and IL-4.
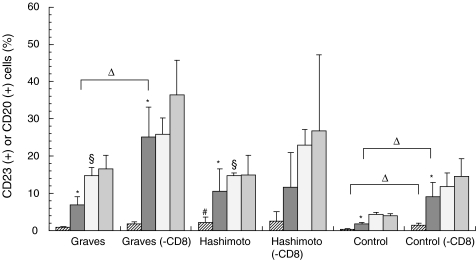
Fig. 2.
The percentages of CD23+ or CD20+ cells in PBMC or CD8-depleted cells (– CD8) from individuals with Graves’ disease or Hashimoto’s thyroiditis, and normal controls. The PBMC or CD8-depleted cells were incubated in the presence or absence of anti-CD40 mAb and IL-4. *P < 0·05 versus (−); #P < 0·05 versus Graves or control; ▵P < 0·05 versus -CD8; §P < 0·05 versus control.  , CD23 (−);
, CD23 (−);  , CD23 (anti-CD40 antibody and IL-4);
, CD23 (anti-CD40 antibody and IL-4);  , CD20 (-);
, CD20 (-);  , CD20 (anti-CD40 and IL-4).
, CD20 (anti-CD40 and IL-4).
Incubation of PBMC with anti-CD40 monoclonal antibodies and IL-4 significantly increased the levels of sCD23 secreted by Graves’ disease patients’ cells in vitro (from 2·5 ± 0·4–26·6 ± 9·7 ng/ml, P < 0·05), Hashimoto’s thyroiditis (from 1·6 ± 0·2–10·2 ± 4·7 ng/ml, P < 0·05), as well as normal controls (from 1·6 ± 0·3–13·9 ± 4·4 ng/ml, P < 0·05). Depletion of CD8+ cells did not affect this anti-CD40/IL-4-induced increased sCD23 in patients with Graves’ disease (from 0·9 ± 0·3–44·5 ± 23·6 ng/ml, P < 0·05), or normal controls (from 0·9 ± 0·3–4·0 ± 1·3 ng/ml, P < 0·05) (Fig. 1). Thus, there was no statistically significant difference between sCD23 levels after stimulation whether or not CD8+ cells were depleted.
Incubation of PBMC with anti-CD40 monoclonal antibodies and IL-4 significantly increased the levels of CD23+ cells in patients with Graves’ disease (from 0·9 ± 0·2–7·0 ± 2·2%, P < 0·05), Hashimoto’s thyroiditis (from 2·2 ± 1·4–10·6 ± 6·1%, P < 0·05), and normal controls (from 0·4 ± 0·1–1·8 ± 0·4%, P < 0·05). Incubation of CD8-depleted cells with anti-CD40/IL-4 increased CD23+ cell levels more markedly in patients with Graves’ disease (from 1·8 ± 0·6–25·1 ± 8·1%, P < 0·05), as well as in normal controls (from 1·5 ± 0·5–9·2 ± 3·7%, P < 0·05), compared with responses of CD8-replete PBMC. No further increase was observed in the CD23+ levels in Hashimoto’s thyroiditis. The basal percentages of CD23+ cells in Hashimoto’s thyroiditis were higher than those in Graves’ disease and normal controls (Fig. 2).
The percentage of CD20+ cells was greater in unstimulated PBMC from patients with Graves’ disease and Hashimoto’s thyroiditis than in normal controls. There were no increases after stimulation with anti-CD40 antibody and IL-4 (Fig. 2). These data suggest that stimulation with anti-CD40 antibody and IL-4 mainly activates CD23+ cells among the B cell populations.
Effect of the presence or absence of CD8+ cells on anti-CD40 antibody and IL-4-induced production of IL-10 (Figs 3 and 4)
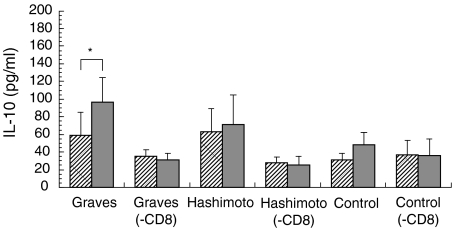
Fig. 3.
The production of IL-10 by PBMC or CD8-depleted cells (– CD8) from individuals with Graves’ disease or Hashimoto’s thyroiditis, and normal controls. The PBMC or CD8-depleted cells were incubated in the presence or absence of anti-CD40 MoAb and IL-4. *P < 0·05.  , (−);
, (−);  , anti-CD40 antibody and IL-4.
, anti-CD40 antibody and IL-4.
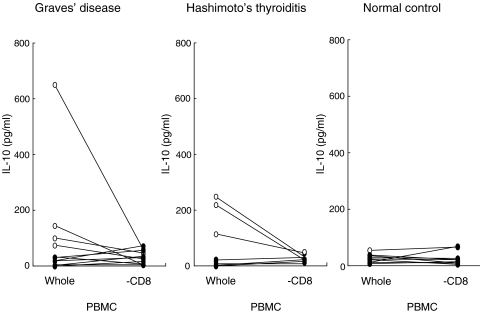
Fig. 4.
The effect of CD8+ cell depletion on the basal secretion of IL-10 by PBMC and CD8-depleted cells from patients with Graves’ disease or Hashimoto’s thyroiditis, and normal controls. Comparison between the group with high basal values (○) and those with low basal values (•) is shown.
Incubation of PBMC with anti-CD40 monoclonal antibodies and IL-4 significantly stimulated the release of IL-10 in patients with Graves’ disease (from 59·0 ± 25·7–96·5 ± 28·2 pg/ml, P < 0·05). This increase was prevented by CD8 depletion. Moreover, PBMC from patients with Hashimoto’s thyroiditis or controls did not show significant changes in IL-10 secretion in response to anti-CD40 antibody and IL-4 (Fig. 3). Graves’ patients could be divided into two groups on the basis of the constitutive IL 10 production by their PBMC. The high group produced > 35 pg/ml, while the low group produced <34 pg/ml (Fig. 4). In the latter, basal production of IL-10 was increased from 15·1 ± 5·2 pg/ml to 38·7 ± 10·2 pg/ml after CD8+ cell depletion. In contrast, in the high release group, IL-10 levels were decreased from 190·0 ± 116·5 pg/ml to 31·0 ± 11·1 pg/ml (Fig. 4) by CD8+ cell depletion. Applying this IL-10 secretion cut-off level to other diseases, it was found that there was also an increase of basal IL-10 release after CD8+ cell depletion from 11·9 ± 7·6 pg/ml to 17·8 ± 7·5 pg/ml in the low group and a decrease from 190·0 ± 40·5 pg/ml to 34·3 ± 8·1 pg/ml in the high group in Hashimoto’s thyroiditis as well (Fig. 4). On the other hand, in the normal control low release group, only 25% of subjects showed an increase, and the remaining 75% a decrease of basal IL-10 release after CD8+ cell depletion (P < 0·05 versus Graves’ disease; Fig. 4). Therefore, this suppression of basal release by CD8+ cells is characteristic of autoimmune thyroid diseases.
DISCUSSION
The pathophysiology of autoimmune disease is related closely to a loss of balance between Th1 and Th2 responses. In Graves’ disease, Th2 cells involved in humoral immunity are possibly dominant, because anti-TSH receptor antibodies contribute to the aetiology of this disease. Several studies including our own have reported that (1): B-cell growth factors are increased [5] not only in the thyroid-infiltrating cells but also in PBMC (2); CD4+CD45RO+CD29+ memory T cells helping to activate B cells account for a large fraction of the thyroid-infiltrating lymphocytes, which are counteracted by CD8+ T cells [5]; (3); CD23+ cells [6] and soluble CD23 [7] are increased in PBMC derived from Graves’ disease patients; and (4) Th2 cells, by secreting IL-4, IL-5 and IL-6, provide more efficient help for B-cell activation, antibody production, and switching to the IgE and IgG1 isotypes [17,18]. Moreover, Graves’ patients with high levels of serum IgE tend to resist antithyroid drug-mediated reductions in anti-TSH receptor antibody titres [19]. Recurrence or onset of Graves’ disease after the onset of allergic rhinitis has also been reported [20,21]. These findings support the pathogenetic roles of Th2 cells, at least in part, in exacerbating Graves’ disease. In untreated Graves’ patients, the number of CD8+ cells is decreased, but activated (DR-positive) CD8+ cells are increased again with hyperthyroidism [14]. These findings suggest the possibility that activated CD8+ T cells may correct the Th1/Th2 balance of Graves’ disease by reducing Th2 dominance. Those data also suggest that the findings obtained using PBMC, at least in part, represent an ongoing process in organ-specific autoimmune thyroid disease.
It has been noted that Graves’ disease is often ameliorated during pregnancy, in which a shift from Th1 to Th2 occurs [22]. In pregnancy, activated CD8+ cells are increased compared to CD4+ cells [23,24] a similar situation to that which has been observed in treated Graves’ patients [14]. This increase of CD8 cells may counteract the dominance of Th2 in pregnant Graves’ disease patients. Moreover, the existence of microchimerism in pregnancy may modify the immune response in a complex manner [25].
Cross-linking with anti-CD40 antibody activates B cells, resulting in cytokine production, including IL-10 [16]. Because IL-10 inhibits the production of cytokines from Th1 cells [26], while activating B cells [27] and activated B cells do not produce IL-12 [28], Th2 cells are preferentially activated by B cell APC through the collaboration of CD40 and IL-4. Increased secretion of sCD23 and increased numbers of CD23+ cells induced by activated B cells via anti-CD40 antibody and IL-4 promoted IL-10 production, especially in PBMC from patients with Graves’ disease, reflecting a dominance of Th2 cells. An increase of CD23+ cells after depletion of CD8+ cells may provide evidence that CD8+ cells suppress activation of B cells. In our studies, the mean degree of depletion of CD8+ cells was estimated at about 45%. This reduction of CD8+ cells by aproximately half resembles the clinical situation and our previous in vitro study (1): the reduction of CD8+ cells in hyperthyroid Graves’ disease was about 27%[14]; and (2) stimulation with anti-CD40 antibody and IL-4 decreased CD8+ cells by 49%[15]. Secretion of IL-10 increased after depletion of CD8+ cells in a group of patients with low basal secretion of IL-10, while it was decreased reciprocally in a group of patients with high basal secretion. Similar changes were observed in the thyroid-infiltrating cells from the excised thyroid of the present Graves’ patient (data not shown). Therefore, these changes may reflect partly the changes occurring in thyroid tissue, the target for organ-specific autoimmune thyroid diseases. These results suggest that CD8+ cells inhibit the induction of Th2 cells per se in about half of the patients in this study. In contrast, CD8+ cells could not inhibit the induction of Th2 cells in the presence of high levels of IL-10. There was no difference between the high and low basal secretion groups as far as clinical findings are concerned. Heuer et al.[29] reported that patients with Graves’ disease could be subdivided into two groups according to their serum levels of anti-TPO antibodies. Patients with an anti-TPO antibody titre of more than 4000 U/ml showed greater T cell infiltration and higher levels of IL-4 and IL-10 mRNA in their thyroid tissues compared to those with antibody of less than 200 U/ml. Applying this criterion to the present study, our cases all registered less than 81 U/ml of anti-TPO antibody; thus, none belonged to the high anti-TPO antibody group as defined in Heuer’s study. Further studies are required to clarify these differences in more detail. The number of CD23+ cells was significantly increased by B cell activation through anti-CD40 antibody and IL-4, while the number of CD20+ cells, a pan-B cell marker, was not increased by stimulation in this way, although the percentages of B cells in patients with Graves’ disease and Hashimoto’s thyroiditis were higher than in the control group (Fig. 2). It remains possible that specific subsets of B cells may be stimulated with these agents. Because CD23 is expressed on B2 cells, this stimulation is considered mainly to activate B2 cells. However, further studies are required to clarify the exact mechanism.
The above results indicate that stimulation via anti-CD40 antibody and IL-4 activates CD23+ B cells and enhances the induction of Th2 cells. In Graves’ disease, Th2 cells are induced strongly by B cell activation via a CD40 pathway, resulting in overproduction of IL-10, and CD8+ cells are considered to inhibit this series of responses. Thus, enhancement of CD8+ cells may become a reasonable treatment for correcting a shift in the Th1/Th2 balance towards Th2 dominance in Graves’ disease.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (Grant-in-Aid for Scientific Research (c) no. 09671081) and from Fujita Health University. The authors thank Ms N. Takekawa for her secretarial assistance.
REFERENCES
- 1.Weetman AP, McGregor AM. Autoimmune thyroid disease: developments in our understanding. Endocr Rev. 1984;5:309–55. doi: 10.1210/edrv-5-2-309. [DOI] [PubMed] [Google Scholar]
- 2.Smith BR, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988;9:106–21. doi: 10.1210/edrv-9-1-106. [DOI] [PubMed] [Google Scholar]
- 3.DeGroot LJ, Quintans J. The causes of autoimmune thyroid disease. Endocr Rev. 1989;10:537–62. doi: 10.1210/edrv-10-4-537. [DOI] [PubMed] [Google Scholar]
- 4.Pozzilli P, Carotenuto P, Delitala G. Lymphocytic traffic and homing into target tissue and the generation of endocrine autoimmunity. Clin Endocrinol. 1994;41:545–54. doi: 10.1111/j.1365-2265.1994.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 5.Goto Y, Itoh M, Ohta Y, Ogawa N, Goto Y, Ohashi H. Increased production of B-cell growth factor by T lymphocytes in Graves’ thyroid: possible role of CD4+ CD29+ cells. Thyroid. 1997;7:567–73. doi: 10.1089/thy.1997.7.567. [DOI] [PubMed] [Google Scholar]
- 6.Corrales JJ, Orfao A, López A, Ciudad J, Mories MT. Serial analysis of the effects of methimazole therapy on circulating B cell subsets in Graves’ disease. N Engl J Med. 1996;287:421–5. doi: 10.1677/joe.0.1510231. [DOI] [PubMed] [Google Scholar]
- 7.Sayinalp S, Akalin S, Sayinalp N, et al. Serum immunoglobulin E and soluble CD23 in patients with Graves’ disease. Horm Metab Res. 1996;28:133–7. doi: 10.1055/s-2007-979145. [DOI] [PubMed] [Google Scholar]
- 8.Noelle RJ, Ledbetter JA, Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992;13:431–3. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 10.Kallmann BA, Hüther M, Tubes M, et al. Systemic bias of cytokine production toward cell-mediated immune regulation in IDDM and toward humoral immunity in Graves’ disease. Diabetes. 1997;46:237–43. doi: 10.2337/diab.46.2.237. [DOI] [PubMed] [Google Scholar]
- 11.Mullins RJ, Cohen SBA, Webb LMC, et al. Identification of thyroid stimulating hormone receptor-specific T cells in Graves’ disease thyroid using autoantigen-transfected Epstein–Barr virus-transformed B cell lines. J Clin Invest. 1995;96:30–7. doi: 10.1172/JCI118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paschke R, Schuppert F, Taton M, Velu T. Intrathyroidal cytokine gene expression profiles in autoimmune thyroiditis. J Endocrinol. 1994;141:309–15. doi: 10.1677/joe.0.1410309. [DOI] [PubMed] [Google Scholar]
- 13.Watson PF, Pickerill AP, Davies R, Weetman AP. Analysis of cytokine gene expression in Graves’ disease and multinodular goiter. J Clin Endocrinol Metab. 1994;79:355–60. doi: 10.1210/jcem.79.2.8045947. [DOI] [PubMed] [Google Scholar]
- 14.Ohashi H, Okugawa T, Itoh M. Circulating activated T cell subsets in autoimmune thyroid disease. Differences between untreated and treated patients. Acta Endocrinol (Copenh) 1991;125:502–9. doi: 10.1530/acta.0.1250502. [DOI] [PubMed] [Google Scholar]
- 15.Itoh M, Uchimura K, Hayakawa N, et al. Surface expression and release of soluble forms of CD8 and CD23 in CD40- and IL-4-activated mononuclear cells from patients with Graves’ disease (GD) Clin Exp Immunol. 1998;113:309–14. doi: 10.1046/j.1365-2249.1998.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh M, Uchimura K, Makino M, et al. Production of IL-10 and IL-12 in CD40 and interleukin-4-activated mononuclear cells from patients with Graves’ disease. Cytokine. 1900;12:688–93. doi: 10.1006/cyto.1999.0659. [DOI] [PubMed] [Google Scholar]
- 17.Stevens TL, Bossie A, Sanders VM, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh C-S, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–9. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada T, Sato A, Komiya I, et al. An elevation of serum immunoglobulin E provides a new aspect of hyperthyroid Graves’ disease. J Clin Endocrinol Metab. 1900;85:2775–8. doi: 10.1210/jcem.85.8.6741. [DOI] [PubMed] [Google Scholar]
- 20.Hidaka Y, Amino N, Iwatani Y, Itoh E, Matsunaga M, Tamaki H. Recurrence of thyrotoxicosis after attack of allergic rhinitis in patients with Graves’ disease. J Clin Endocrinol Metab. 1993;77:1667–70. doi: 10.1210/jcem.77.6.8263157. [DOI] [PubMed] [Google Scholar]
- 21.Hidaka Y, Masai T, Sumizaki H, Takeoka K, Tada H, Amino N. Onset of Graves’ thyrotoxicosis after an attack of allergic rhinitis. Thyroid. 1996;6:349–51. doi: 10.1089/thy.1996.6.349. [DOI] [PubMed] [Google Scholar]
- 22.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is succesful pregnancy a Th2 phenomenon. Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe M, Iwatani Y, Hidaka Y, Mitsuda N, Amino N. Changes in soluble CD4 and CD8 proteins in healthy pregnant and postpartum women. Am J Reprod Immunol. 1996;36:220–7. doi: 10.1111/j.1600-0897.1996.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 24.Mikyas Y, Aziz N, Harawa N, et al. Immunologic activation during pregnancy. serial measurement of lymphocyte phenotype and serum activation molecules in HIV-infected and uninfected women. J Reprod Immunol. 1997;33:157–70. doi: 10.1016/s0165-0378(97)00018-1. [DOI] [PubMed] [Google Scholar]
- 25.Klintschar M, Schwaiger P, Mannweiler S, Regauer S, Kleiber M. Evidence of fetal microchimerism in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 1901;86:2494–8. doi: 10.1210/jcem.86.6.7540. [DOI] [PubMed] [Google Scholar]
- 26.Hino A, Nariuchi H. Negative feedback mechanism suppresses interleukin-12 production by antigen-presenting cells interacting with T helper 2 cells. Eur J Immunol. 1996;26:623–8. doi: 10.1002/eji.1830260318. [DOI] [PubMed] [Google Scholar]
- 27.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–3. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adorini L, Guéry J-C, Ria F, Galbiati F. B cells present antigen to CD40+ T cells, but fail to produce IL-12: selective APC for Th2 cell development? Ann NY Acad Sci. 1997;815:401–11. doi: 10.1111/j.1749-6632.1997.tb52091.x. [DOI] [PubMed] [Google Scholar]
- 29.Heuer M, Aust G, Ode-Hakim S, Scherbaum WA. Different cytokine mRNA profiles in Graves’ disease, Hashimoto’s thyroiditis, and nonautoimmune thyroid disorders determined by quantitative reverse transcriptase polymerase chain reaction (RT-PCR) Thyroid. 1996;6:97–106. doi: 10.1089/thy.1996.6.97. [DOI] [PubMed] [Google Scholar]