Abstract
Seeking better immunological markers indicating the long-term outcome of cystic echinococcosis (CE) after chemotherapy we studied 23 patients receiving albendazole, clinically followed for 8 years, and grouped ultrasonographically according to therapeutic outcome. Antibody responses against a partially purified fraction of hydatid fluid (HFF) and antigen B (AgB) were evaluated by indirect haemagglutination (IHA), ELISA and immunoblotting (IB). Although IHA titres varied over the course of treatment, differences in mean antibody titres to HFF between groups were significant only at 4 years (P = 0·031). IgG isotype expression remained unchanged during follow-up whereas IgE expression decreased in patients with cured or stable disease. AgB disclosed higher IgG4 expression (P < 10−4; P = 0·025) and lower IgG1 expression than HFF (P < 10−4; P = 0·022). IHA antibody titres were higher in patients with progressive than in those with cured or stable disease, even in those with the same cyst type. ELISA isotype profiles differed between groups, particularly for type CE 3, 4 and 5 cysts: higher serum IgG1 and IgG3, lower IgG4 and IgE in patients with cured or stable disease. Although combined serological testing provides scarce information on the long-term outcome of CE after chemotherapy it may be useful for reviewing in a retrospective study the outcome of a cyst and for assessing the host-parasite relationship
Keywords: cystic echinococcosis, immunological markers, chemotherapy, long-term follow-up, immunological tests
INTRODUCTION
Cystic echinococcosis (CE) caused by Echinococcus granulosus, an important public health problem in many parts of the world, remains silent for years before the enlarging cysts cause symptoms in the affected organs. Although most patients with hydatid cysts undergo surgery, removal of the parasite mass rarely solves the problem. Because benzimidazole carbamates (mebendazole and albendazole) have proved effective against the larval stages of E. granulosus in humans, chemotherapy, once reserved for inoperable cases of CE, is now more widely used [1]. The response to treatment is unpredictable; it also entails constant medical supervision and regular monitoring of imaging findings and serological responses. The incidence of relapse increases with the length of follow-up [2]. Monitoring imaging findings during follow-up can be difficult because cysts often undergo relatively small changes that imaging cannot visualize. The viability and presence of all foci is also difficult to assess [3]. As a method for clinical follow-up, serological testing also has drawbacks because specific antibodies may persist in patients’ sera for several years after recovery [1]. Among the newer serological tests for assessing whether an infection will progress or regress, assay of immunoglobulin isotypes with the use of distinct parasite antigens seems an interesting new approach [4,5]. Parasitic proteins that have the major immunodiagnostic value in detecting E. granulosus are antigen 5 and antigen B (AgB). Although much immunological evidence suggests that AgB has a high diagnostic value, its importance in monitoring the effectiveness of pharmacological treatment in CE is unknown [6–8]
An effective serological method for long-term monitoring after chemotherapy for CE is therefore, still lacking. This immunological study was designed to assess the usefulness of long-term serological monitoring by antibody detection in the clinical management of patients with cystic echinococcosis. We studied a series of 23 patients all of whom received albendazole therapy for CE and completed an imaging and serological follow-up lasting eight years. Patients were divided into two groups according to the outcome of chemotherapy as evaluated by ultrasonographic (US) imaging of the cysts. Before chemotherapy, 4 years later, and at 8 years, sera were assayed for total IgG, IgG subclasses and IgE using AgB and a partially purified fraction of hydatid fluid, in combined immunological tests
MATERIALS AND METHODS
Antigens
Sheep hydatid fluid was collected from fertile cysts, clarified by centrifugation at 10 000g for 60min at 4°C and kept at −20°C for subsequent use. Two antigen preparations were used: a partially purified hydatid fluid fraction (HFF), rich in antigen 5 and antigen B, obtained from crude sheep hydatid fluid by precipitation at low ionic strength (0·005m acetate buffer, pH 5) according to Oriol et al.[9]; and antigen B (AgB) obtained after heating HFF at 100°C as described by Rogan et al.[10]
Patients and cysts
From January 1992 until December 2000, in the II Chair of Infectious Disease of the Department of Infectious and Tropical Disease, University of Rome ‘La Sapienza’ 23 consecutive patients with clinically proven CE located in various body organs (16 with cysts in the liver, 4 with cysts in the muscle and liver and 3 with cysts in the lung) underwent chemotherapy for hydatidosis (Table 1). All of them received 3–6 month cycles of albendazole 10–12 mg/kg per day sometimes repeated according to the World Health Organization protocol [11]. During therapy, all patients underwent clinical, blood chemical, immunological and US assessment. As an index of albendazole-induced degeneration of the hydatid cysts, we assessed the following morphological changes: volumetric reduction (>10% until disappearance), detachment of parasitic membranes, pseudo- solidification, reduction or disappearance of daughter cysts, and calcification. Hydatid cysts were classified into five types according to the the sonographic classification proposed by the WHO Informal Group on Echinococcosis: type CE1 (unilocular, simple cysts), type CE2 (multivescicular, multiseptated cysts), type CE3 (unilocular cyst which may contain daughter cysts with detachment of laminated membrane), type CE4 (heterogeneous or hyperechoic degenerative contents) and type CE5 (calcified cysts) [12]. Types CE1, and CE2 represent the active and progressive phase of CE; type CE3 represents a transitional stage which usually starts to degenerate, and types CE4 and 5 represent the regressive phase characterized by infiltration, degeneration and calcification. The 23 patients were divided in two groups according to the clinical efficacy of treatment. Seven patients had cured or stable disease (stable success of therapy, without relapse): their hydatid cysts had disappeared, solidified or calcified; and 16 patients had progressive disease (unsuccessful of therapy or reactivation of parasitosis once or more times), their hydatid cysts had briefly regressed then relapsed so that new cysts appeared or the liquid component of the matrix increased and membrane detachment disappeared. All patients were followed for eight years and the 69 serum samples included in the study were collected before chemotherapy (T0), at 4 years after chemotherapy began (T1) and at the end of follow-up (T2). After collection, the sera were divided into aliquots and kept at −20°C until use. All procedures were approved by the local Ethical Committee, and all subjects gave their informed consent to the study
Table 1.
Clinical features of the 23 patients with cystic echinococcosis
| Cyst type* | |||||||
|---|---|---|---|---|---|---|---|
| Patient no | Age/Sex | Cyst location | T0** | T1 | T2 | Previous surgery | Albendazole cycle |
| Patients with cured or stable disease | |||||||
| 1 | 21/M | Liver | CE 2 | CE 5 | CE 5 | Yes | 1 |
| 2 | 64/F | Lung | CE 2 | CE 5 | CE 5 | Yes | 2 |
| 3 | 54/M | Multiple† | CE 1 | CE 3 | CE 4 | Yes | 2 |
| 4 | 44/F | Liver | CE 1 | CE 5 | CE 5 | Yes | 2 |
| 5 | 32/M | Liver | CE 3 | CE 4 | CE 4 | Yes | 1 |
| 6 | 48/M | Multiple | CE 1 | CE 5 | CE 5 | Yes | 1 |
| 7 | 54/M | Liver | CE 1 | CE 5 | CE 5 | No | 1 |
| Patients with progressive disease | |||||||
| 8 | 55/M | Lung | CE 1 | CE 1 | CE 5 | Yes | 3 |
| 9 | 23/M | Multiple | CE 2 | CE 5 | CE 5 | Yes | 2 |
| 10 | 40/F | Liver | CE 1 | CE3 | CE 5 | Yes | 2 |
| 11 | 33/F | Liver | CE 2 | CE 3 | CE 4 | Yes | 4 |
| 12 | 76/F | Liver | CE 2 | CE 3 | CE 2 | Yes | 5 |
| 13 | 34/M | Liver | CE 2 | CE 2 | CE 4 | No | 3 |
| 14 | 57/M | Liver | CE 2 | CE 2 | CE 4 | No | 8 |
| 15 | 60/M | Liver | CE 2 | CE 3 | CE 2 | No | 3 |
| 16 | 65/F | Liver | CE 1 | CE 2 | CE 4 | No | 10 |
| 17 | 35/M | Liver | CE 2 | CE 2 | CE 5 | Yes | 2 |
| 18 | 49/M | Liver | CE 2 | CE 2 | CE 5 | No | 3 |
| 19 | 31/F | Liver | CE 1 | CE 1 | CE 1 | Yes | 2 |
| 20 | 58/M | Multiple | CE 1 | CE 2 | CE 4 | Yes | 10 |
| 21 | 66/M | Lung | CE 2 | CE 2 | CE 4 | No | 5 |
| 22 | 64/F | Liver | CE 2 | CE 3 | CE 4 | Yes | 4 |
| 23 | 36/F | Liver | CE 1 | CE 2 | CE 1 | No | 4 |
According to the sonographic classification proposed by the WHO Informal Group on Echinococcosis [12].
Samples included in the study were collected before chemotherapy (T0) at 4 years after chemotherapy began (T1) and at the end of follow-up (T2).
Multiple location indicates cysts in the muscle and in the liver.
Serological tests
Serum samples were tested for antibody detection by indirect haemagglutination (IHA) as described by Bombardieri et al.[13]. In brief, sheep blood cells were activated with tannic acid and then coated with HFF (2 mg/ml) and AgB (1 mg/ml) and used at 5% in phosphate buffered saline pH 7·2 as final concentration. Sera retaining their agglutinative ability in dilutions higher than 1:160 were considered positive. Patients’ sera were tested by enzyme linked immunosorbent assay (ELISA) using either the HFF (2 μg/well) as described by Iacona et al.[14], or AgB (5 γ/well) as described by Ioppolo et al.[8]. Human sera were diluted 1:2000 for total IgG, 1:500 for IgG subclasses and 1:50 for IgE detection. O-phenylenediamine dihydrochloride (Sigma Chemical Co. St Louis, MO) was used as substrate. Optical densities (OD) at 490 nm were considered positive when higher than 0·3 for IgE, 0·06 for IgG1, 0·007 for IgG2, 0·01 for IgG3 and 0·003 for IgG4 when the antigen was HFF and higher than 0·3 for IgE, 0·03 for IgG1, 0·003 for IgG2, 0·02 for IgG3 and 0·004 for IgG4 when the antigen was AgB (mean + 2 SD of absorbance readings in uninfected controls). The reproducibility of the results was monitored by including in each ELISA plate negative serum samples in triplicate and three positive serum samples, each yielding different OD values
Patients’ sera were tested by immunoblotting (IB) as previously described by Siracusano et al.[15]. HFF and AgB were run in SDS-PAGE in a 12% gel under nonreducing conditions. The antigenic subunits at 65 and 55kD correspond to antigen 5, the subunit at 8/12 kD corresponds to AgB. Human sera were diluted 1:500 for total IgG, 1:50 for IgG subclasses and 1:10 for IgE detection. 3,3′-didiaminobenzidine dihydrochloride (Sigma Chemical Co.) was used as substrate
Antisera
A goat antihuman IgG horseradish peroxidase conjugate serum (Bio-Rad, Richmond, CA, USA) was used as second serum to determine total IgG, and a goat antihuman IgE peroxidase labelled serum (Cappel, Cochranville, PA, USA) to determine IgE. Monoclonal mouse antihuman IgG1, IgG2, IgG3, or IgG4 (BD PharMingen Co, CA, USA) were used to determine IgG subclasses and a goat antimouse IgG peroxidase labelled serum (Bio-Rad) was used as second serum. Two monoclonal antibodies, E5 specific for antigen 5 and A11B1 specific for AgB, previously prepared in our laboratory, were used as controls to identify antigenic subunits [16,17]
Protein measurement
Protein concentrations were estimated using the Bio-Rad Bradford protein assay kit (Bio-Rad) and bovine plasma gamma globulin as a standard
Statistical analysis
Student's t-test was used to compare differences between arithmetic means. P-values equal to or less than 0·05 were considered to indicate statistical significance
RESULTS
Antibody determination by IHA
HFF and AgB antibody titres were invariably lower in patients with cured or stable disease than in patients with progressive disease. Differences in mean antibody titres to HFF between the two clinical groups reached statistical significance only at T2 (4 years) (P = 0·031) (Fig. 1). Although IHA titres varied over the course of treatment in both clinical groups, they progressively decreased only in patients with cured or stable disease
Fig. 1.
Antibody response determined by indirect haemagglutination in the 23 patients with cystic echinococcosis pharmacologically treated with albendazole and grouped according to the outcome of chemotherapy. Serum samples were collected before chemotherapy (□), at 4 years after chemotherapy began ( ) and at the end of follow-up (▪). Student's t-test was used to compare differences between arithmetic means. *P = 0·031
) and at the end of follow-up (▪). Student's t-test was used to compare differences between arithmetic means. *P = 0·031
Antibody determination by ELISA
ELISAs determining isotype antibody expression in response to HFF and AgB, showed no significant variations between or within groups during the long-term follow-up (Fig. 2). In both antigen ELISAs, IgE levels alone decreased more evidently in patients with cured or stable disease than in patients with progressive disease. AgB elicited lower mean ELISA ODs than HFF for all subclasses except IgG4 and IgE. Mean ODs at T0 and T1 in patients with progressive disease were significantly higher for IgG4 in response to AgB than for IgG4 in response to HFF (P < 10−4; P = 0·025). Conversely, in all three samples from both groups, mean ODs were significantly higher for IgG1 in response to HFF than for IgG1 in response to AgB (P < 10−4; P = 0·022)
Fig. 2.
Antibody isotype pattern determined by ELISA in the 23 patients with cystic echinococcosis pharmacologically treated with albendazole and grouped according to the outcome of chemotherapy. Serum samples were collected before chemotherapy (□), at 4 years after chemotherapy began ( ) and at the end of the 8-year follow-up (▪). Student's t-test was used to compare differences between arithmetic means. *P < 10−4; †P = 0·022; ‡P = 0·025. Data represent means ± 2 SD of triplicate experiments.
) and at the end of the 8-year follow-up (▪). Student's t-test was used to compare differences between arithmetic means. *P < 10−4; †P = 0·022; ‡P = 0·025. Data represent means ± 2 SD of triplicate experiments.
Qualitative antibody analysis by IB
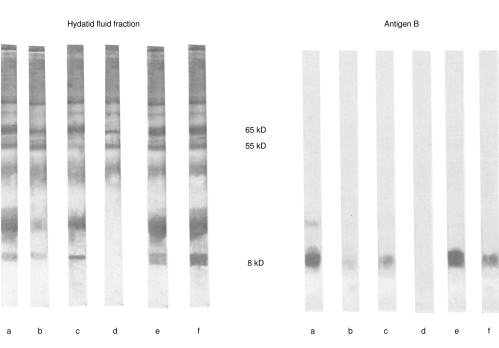
Positive reactions recognized by IB showed a positive association with antibody isotype responses as measured by HFF- and AgB-ELISA (data not shown). In both groups of patients, IB analysis disclosed preferential binding of the IgG1, and IgG3 subclasses to 55–65 kD subunits of HFF and of IgG4 and IgE to 8-, 16-, and 20-kD of AgB (Fig. 3)
Fig. 3.
Immunoblotting after 12% SDS-PAGE of hydatid fluid fraction and antigen B under non reducing conditions revealed with antisera specific to total IgG (lane a), IgG1 (lane b), IgG2 (lane c), IgG3 (lane d), IgG4 (lane e), IgE (lane f).
Relationship between serological response and US cyst classification
When we compared IHA serum antibody titres with the US classification of the cysts, HFF and AgB antibody titres were higher in patients with progressive disease than in those with cured or stable disease, even in patients from the two groups who had the same cyst type (Fig. 4). Again, the relationship between the isotype profiles in ELISA and cyst type differed in the two groups of patients, particularly the association with cysts of type CE 3, 4 and 5. In response to both antigens, and especially to AgB, ELISA detected higher mean ODs for the IgG3 subclass and lower ODs for IgG4 in sera from patients with cured or stable disease than in those with progressive disease (Fig. 5)
Fig. 4.
Antibody response determined by indirect haemagglutination in the 23 patients with cystic echinococcosis pharmacologically treated with albendazole and grouped according to the type of cyst as classified by ultrasonographic scans in accordance with the WHO Informal Group on Echinococcosis [12]. Differences between arithmetic means did not result statistically significant when tested by Student's t-test. □ Patients with cured or stable disease; ▪ Patients with progressive disease.
Fig. 5.
Antibody isotype pattern determined by ELISA in the 23 patients with cystic echinococcosis pharmacologically treated with albendazole divided according to the type of cyst as classified by ultrasonographic scans in accordance with the WHO Informal Group on Echinococcosis [12]. Differences between arithmetic means did not result statistically significant when tested by Student's t-test. Note the higher IgG1 and IgG3 in stable disease and the higher IgG4 and IgE in progressive disease. Data represent means ± 2 SD of triplicate experiments. □ Patients with cured or stable disease; ▪ Patients with progressive disease.
DISCUSSION
Whereas numerous studies report the usefulness of immunosurveillance in the follow-up of patients with CE after surgery [18–20], this study, to our knowledge, is one of the few reporting the use of immunosurveillance in the long term follow-up of patients with CE after chemotherapy
The findings from this study of patients with CE undergoing chemotherapy with albendazole suggest that long-term serological monitoring provides limited support to diagnostic imaging techniques in assessing the outcome of chemotherapy. But it promises to be useful for studying in depth the complex immunological mechanisms that influence the outcome of chemotherapy. Because haemagglutinating antibodies and IgE decrease as therapy induces benefits combined assessment of these two immunological markers may give additional information on the long-term infection status. IgG antibodies retain floating levels for many years even after ‘cure’ they cannot be considered as immunological markers of the outcome of therapy. Expression of the various IgG isotypes, which remain practically unchanged over the 8-year follow-up, before therapy differed in the patients grouped according to the outcome of chemotherapy. IgG isotype expression differed also in its HFF and AgB binding profiles. Hence although IgG isotypes cannot be considered as immunological markers of the outcome of chemotherapy, they may be a useful guide to the clinical management of CE
The human immune system reacts differently to chemotherapy and surgery. Surgical removal of cysts eliminates the invasive parasite and its multiple manifestations, thus leading to recovery. The lack of parasitic antigenic material causes antibody titres, especially those of IgE, to decrease rapidly [21]. Albendazole inhibits the assembly of tubulin into microtubules, thus impairing glucose uptake and interfering with parasite homeostasis [22]. Albendazole also exerts a more potent action in some cysts (or patients) than in others, sometimes acting exclusively as a parasitostatic agent thus allowing the parasite to remain in situ. Another problem facing patients and physicians is the relapse of hydatid cysts after chemotherapy. Evaluating the results obtained during a long-term follow-up (at least 1 year) in a series of patients with CE treated with benzimidazole carbamates, Franchi et al.[2] observed a ~25% of relapse of treated cysts. Hence they underlined the need for long-term clinical and imaging follow-up. Although the persistence of parasite in the organism and the state of dynamic equilibrium between the parasite and the host affect the immune response, in sequential samples of patients’ sera they induce only small variations in specific antibodies. For these reasons, no serological method so far tested – changes in specific antibody titres, phase-dependent antigens, specific immune complexes, or multiple tests combined – has proved fully reliable in predicting the outcome of chemotherapy or a possible cure [23,24]
In the patients with cured or stable disease in this study, antibody titres in response to HFF decreased over the 8-year follow-up; but in patients whose hydatid cysts relapsed, they persisted. This finding supports studies underlining the usefulness of IHA during albendazole therapy [25,26]. Contrary to expectations, given the reported diagnostic usefulness of AgB in IB and ELISA [8,27], AgB in IHA yielded disappointing results, namely decreased sensitivity (before therapy: 97% positive reactions to HFF versus 48% positivity to AgB). Possible reasons are technical problems inherent to the use of AgB in IHA, including low levels of haemagglutinating antibodies to AgB. AgB-IHA is therefore of no use in the diagnosis of primary infection or relapse. In IHA before, during and after chemotherapy, both antigens yielded higher serum antibody titres in patients with progressive disease than in those with cured or stable disease – even though patients had been ultrasonographically classified into the same group. The possible use of IHA titres before treatment as immunological markers of the outcome of therapy awaits confirmation from a study in a larger number of patients with a long-term clinical follow-up
Our finding that all antibody IgG isotypes directed against HFF and AgB persisted in our patients’ sera during follow-up after albendazole therapy confirms a previous report that IgG-ELISA has no place in the management of CE as a tool for assessing therapeutic success [28]. The heterogeneous results seen in our patient population belonging to the same clinical group, might reflect various factors, including antigenic stimulus, host genetic factors, cyst status, and the number of albendazole cycles. In our study, patients with cured or stable disease received no more than one or two cycles of therapy, whereas those with progressive disease underwent from 2 to 10 cycles. If albendazole damages the cyst wall thus rendering it more permeable, then the release of fluid containing antigenic material might well cause distinct antibody isotype titres to rise. The marked fall in IgE isotype titres in our patients with cured or stable disease suggests that unlike the other isotypes we tested, the IgE isotype closely reflects the actions of albendazole on E. granulosus. Our findings therefore confirm that detection of AgB-specific IgE is useful in the long term clinical follow-up of patients with CE after chemotherapy [5,29]
The differential recognition of the two Echinococcus antigens by the IgG isotypes in ELISA, in particular the preferential binding of IgG1 and IgG3 to HFF, and of IgG4 to AgB, has been previously demonstrated by IB in patients with clinically advanced and in those with asymptomatic CE [4,8,28–32]. Our results underline the role of AgB in skewing the immune response towards a Th2 polarization, particularly in patients with relapse. They also support the role of HFF in eliciting a preferentially Th1 activation [29]. Our data on IgG4 again support the reported closer association of the specific IgG4 isotype with nonresponding patients than with those who respond well [5,33,34]
The differential antibody expression (mean OD values) in the five cyst types provides further evidence that AgB intervenes in progressive disease (low IgG1 and IgG3, high IgG4 and IgE) whereas the other antigenic components of HFF intervene in quiescent disease (high IgG1 and IgG3, low IgG4 and IgE). Again, our data confirm the possible protective role of the IgG3 subclass in CE as reported in other parasitoses [5]
Although serological testing using IB combined with IgG1, IgG3 and IgG4 ELISA testing provided no useful information for the long-term immunological follow-up of patients undergoing chemotherapy for CE, it showed unexpected promise as a method for reviewing in a retrospective study the outcome of a cyst and for assessing the host-parasite relationship
The clinical fate of a cyst cannot be predicted by US imaging alone. Cysts classified by US as identical in clinical type may have distinctly differing fates: some will ultimately progress whereas others will regress. Investigating the immunological mechanisms underlying these distinct outcomes is a prerequisite for improving prediction. Our findings indicate that the serological profile associated with cysts of the same ultrasonographic type (type CE 3-4-5) depends on the fate of the cyst: higher IgG1 and IgG3 in stable disease and higher IgG4 and IgE in progressive disease (Fig. 5). Comparative analysis of cytokine profiles and US cyst classification should therefore give useful additional information on human CE
Despite its limited usefulness in the long term follow-up after chemotherapy for CE, serological monitoring by antibody detection seems promising in the clinical management of cystic echinococcosis. Our results highlight the need for close cooperation among clinicians, radiologists and immunologists
Acknowledgments
This work was supported by ISS grant no. 1139/RI
REFERENCES
- 1.Pawlowski ZS. Critical points in the clinical management of cystic echinococcosis: a revised review. In: Andersen FL, Ouhelli H, Kachani M, editors. Compendium on Cystic Echinococcosis. Brigham Young University PrintServices: Provo; 1997. pp. 199–235. [Google Scholar]
- 2.Franchi C, Di Vico B, Teggi A. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin Inf Dis. 1999;29:304–9. doi: 10.1086/520205. [DOI] [PubMed] [Google Scholar]
- 3.Wen H, New RR, Craig PS. Diagnosis and treatment of human hydatidosis. Br J Clin Pharmacol. 1993;35:565–74. doi: 10.1111/j.1365-2125.1993.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen H, Craig PS. Immunoglobulin G subclass responses in human cystic and alveolar echinococcosis. Am J Trop Med Hyg. 1994;51:741–8. doi: 10.4269/ajtmh.1994.51.741. [DOI] [PubMed] [Google Scholar]
- 5.Riganò R, Profumo E, Ioppolo S, et al. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol. 1995;102:281–5. doi: 10.1111/j.1365-2249.1995.tb03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lightowlers MW, Liu DY, Haralambous A, et al. Subunit composition and specificity of the major cyst fluid antigens of Echinococcus granulosus. Mol Biochem Parasitol. 1989;37:171–82. doi: 10.1016/0166-6851(89)90149-7. [DOI] [PubMed] [Google Scholar]
- 7.Leggatt GR, McManus DP. Identification and diagnostic value of a major antibody epitope on the 12 kDa antigen from Echinococcus granulosus (hydatid disease) cyst fluid. Parasite Immunol. 1994;16:87–96. doi: 10.1111/j.1365-3024.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 8.Ioppolo S, Notargiacomo S, Profumo E, et al. Immunological responses to antigen B from Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol. 1996;18:571–8. doi: 10.1046/j.1365-3024.1996.d01-31.x. [DOI] [PubMed] [Google Scholar]
- 9.Oriol C, Williams JF, Perez MV, et al. Purification of lipoprotein antigens of Echinococcus granulosus from sheep hydatid fluid. Am J Trop Med Hyg. 1971;20:569–74. doi: 10.4269/ajtmh.1971.20.569. [DOI] [PubMed] [Google Scholar]
- 10.Rogan MT, Craig PS, Zeyhle E, et al. Evaluation of a rapid dot-ELISA as a field test for the diagnosis of cystic hydatid disease. Trans Royal Soc Trop Med Hyg. 1991;85:773–7. doi: 10.1016/0035-9203(91)90451-4. [DOI] [PubMed] [Google Scholar]
- 11.Informal Working Group on Echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull World Health Organ. 1996;74:231–42. [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlowski ZS, Eckert J, Vuitton DA, et al. Echinococcosis in humans. clinical aspects, diagnosis and treatment. In: Eckert J, Gemmel MA, Meslin FX, Pawlowski ZS, editors. WHO/OIE Manual on echinococcosis in humans and animals, a zoonosis of global concern. Geneva: WHO; 2001. pp. 20–66. [Google Scholar]
- 13.Bombardieri S, Giordano F, Ingrao F, et al. An evaluation of an agar gel diffusion test with crude and purified antigens in the diagnosis of hydatid disease. Bull World Health Organ. 1974;51:525–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Iacona A, Pini C, Vicari G. Enzyme-linked immunosorbent assay (ELISA) in serodiagnosis of hydatid disease. Am J Trop Med Hyg. 1980;9:248–56. doi: 10.4269/ajtmh.1980.29.95. [DOI] [PubMed] [Google Scholar]
- 15.Siracusano A, Ioppolo S, Notargiacomo S, et al. Detection of antibodies against Echinococcus granulosus antigens and their subunits by immunoblotting. Trans Royal Soc Trop Med Hyg. 1991;85:95–102. doi: 10.1016/0035-9203(91)90039-2. [DOI] [PubMed] [Google Scholar]
- 16.Di Felice G, Pini C, Afferni C, et al. Purification and partial characterization of the major antigen of Echinococcus granulosus (antigen 5) with monoclonal antibodies. Mol Biochem Parasitol. 1986;20:133–42. doi: 10.1016/0166-6851(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 17.Ortona E, Siracusano A, Castro A, et al. Use of a monoclonal antobody against the antigen B of Echinococcus granulosus for purification and detection of antigen B. Appl Parasitol. 1995;36:220–5. [PubMed] [Google Scholar]
- 18.Craig PS. Immunodiagnosis of Echinococcus granulosus and a comparison of techniques for diagnosis of canine echinococcosis. In: Andersen FL, Ouhelli H, Kachani M, editors. Compendium on Cystic Echinococcosis. Provo: Brigham Young University Print Services; 1997. pp. 85–118. [Google Scholar]
- 19.Zarzosa MP, Orduna Domingo A, Gutierrez P, et al. Evaluation of six serological tests in diagnosis and postoperative control of pulmonary hydatid disease patients. Diagn Microbiol Infect Dis. 1999;35:255–62. doi: 10.1016/s0732-8893(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 20.Gadea I, Ayala G, Diago MT, et al. Immunological diagnosis of human hydatid cyst relapse: utility of the enzyme-linked immunoelectrotransfer blot and discriminant analysis. Clin Diagn Laboratory Immunol. 2000;7:549–52. doi: 10.1128/cdli.7.4.549-552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aceti A, Pennica A, Teggi A, et al. IgG subclasses in human hydatid disease: prominence of the IgG4 response. Int Arch Allergy Immunol. 1993;102:347–51. doi: 10.1159/000236582. [DOI] [PubMed] [Google Scholar]
- 22.Lacey E. Mode of action of benzimidazole. Parasitol Today. 1990;6:112–5. doi: 10.1016/0169-4758(90)90227-u. [DOI] [PubMed] [Google Scholar]
- 23.Coltorti EA. Standardization and evaluation of an enzyme immunoassay as a screening test for seroepidemiology of human hydatidosis. Am J Trop Med Hyg. 1986;35:1000–5. doi: 10.4269/ajtmh.1986.35.1000. [DOI] [PubMed] [Google Scholar]
- 24.Craig PS. Detection of specific circulating antigen, immune complexes and antibodies in human hydatidosis from Turkana (Kenya) and Great Britain, by enzyme immunoassay. Parasit Immunol. 1986;8:171–88. doi: 10.1111/j.1365-3024.1986.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 25.Matossian RM, Awar GN, Radwan H, et al. Immune status during albendazole therapy for hydatidosis. Ann Trop Med Parasitol. 1992;86:67–75. doi: 10.1080/00034983.1992.11812632. [DOI] [PubMed] [Google Scholar]
- 26.Ravinder PT, Parija SC, Rao KS. Evaluation of human hydatid disease before and after surgery and chemotherapy by demonstration of hydatid antigens and antibodies in serum. J Med Microbiol. 1997;46:859–64. doi: 10.1099/00222615-46-10-859. [DOI] [PubMed] [Google Scholar]
- 27.Ortona E, Rigano R, Margutti P, et al. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol. 2000;22:553–9. doi: 10.1046/j.1365-3024.2000.00336.x. [DOI] [PubMed] [Google Scholar]
- 28.Force L, Torres JM, Carrillo A, et al. Evaluation of eight serological tests in the diagnosis of human echinococcosis and follow-up. Clin Infect Dis. 1992;15:473–80. doi: 10.1093/clind/15.3.473. [DOI] [PubMed] [Google Scholar]
- 29.Riganò R, Profumo E, Bruschi F, et al. Modulation of human immune response by Echinococcus granulosus antigen B and its possible role in evading host defenses. Infect Immun. 2001;69:288–96. doi: 10.1128/IAI.69.1.288-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McVie A, Ersfeld K, Rogan MT, et al. Expression and immunological characterisation of Echinococcus granulosus recombinant antigen B for IgG4 subclass detection in human cystic echinococcosis. Acta Trop. 1997;67:19–35. doi: 10.1016/s0001-706x(97)00056-9. [DOI] [PubMed] [Google Scholar]
- 31.Shambesh MK, Craig PS, Wen H, et al. IgG1 and IgG4 serum antibody responses in asymptomatic and clinically expressed cystic echinococcosis patients. Acta Trop. 1997;64:53–63. doi: 10.1016/s0001-706x(96)00637-7. [DOI] [PubMed] [Google Scholar]
- 32.Daeki AO, Craig P, Shambesh MK. IgG subclass antibody responses and the natural history of hepatic cystic echinococcosis in asymptomatic patients. Ann Trop Med Parasitol. 2000;94:319–28. doi: 10.1080/00034983.2000.11813546. [DOI] [PubMed] [Google Scholar]
- 33.Bonifacino R, Carter SD, Craig PS, et al. Assessment of the immunological surveillance value of humoral and lymphocyte assays in severe human cystic echinococcosis. Trans R Soc Trop Med Hyg. 2000;94:97–102. doi: 10.1016/s0035-9203(00)90455-3. [DOI] [PubMed] [Google Scholar]
- 34.Guerri ML, Davila M, Rodriguez M, et al. Utility of IgG subclasses in the diagnosis and follow up of hydatidosis. Enferm Infect Microbiol Clin. 2000;18:262–6. [PubMed] [Google Scholar]