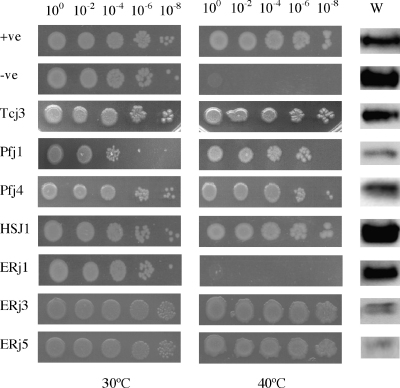
Fig. 3.
All the J-domain chimeras of Agt DnaJ, apart from ERj1-J-Agt-DnaJ, were able to reverse the thermosensitivity of E. coli OD259. Plasmids encoding each of the chimeric Hsp40s were transformed into the temperature sensitive strain E. coli OD259. Cells were diluted sequentially, spotted onto agar plates supplemented with IPTG and grown at the non-stress temperature of 30 °C and stress temperature of 40 °C. The dilution factor is indicated above each growth panel. The ability of each protein to compensate for the lack of DnaJ and CbpA was investigated by monitoring the reversal of thermosensitivity under stress temperature conditions at 40 °C. The proteins produced in the cells are indicated with an abbreviation on the left-hand side of each growth panel: +ve—Agt DnaJ; −ve—Agt DnaJ-H33Q; Tcj3—Tcj3-J-Agt-DnaJ; Pfj1—Pfj1-J-Agt-DnaJ; Pfj4—Pfj4-J-Agt-DnaJ; HSJ1—HSJ1-J-Agt-DnaJ; ERj1—ERj1-J-Agt-DnaJ; ERj3—ERj3-J-Agt-DnaJ; ERj5—ERj5-J-Agt-DnaJ. The levels of chimeric protein production in E. coli OD259 were determined by Western analysis (W).