Patient History
A 43-year-old man presented with a five-year history of a slowly enlarging soft tissue mass in the antecubital region of his left arm. An excisional biopsy of the mass was performed and microscopic examination showed a low-grade spindle cell neoplasm arranged in discrete large nodules of broad sweeping fascicles with a focal filigree pattern, separated by thick bands of tendinous-type fibrous tissue and adipose tissue, with focal cystification (Figure 1A) . Only occasional mitotic figures were noted. A panel of immunostains demonstrated strong diffuse immunoreactivity for vimentin; the stain for epithelial membrane antigen highlighted the focal epithelial component of the tumor (Figure 1 B and C) . Strong immunoreactivity was also present for CD99 and CD57, with focal immunoreactivity for cytokeratin 7, but there was no immunoreactivity for pancytokeratin, cytokeratin 20, or S100 protein. The morphological and immunohistochemical findings supported the diagnosis of synovial sarcoma (SS), and molecular analysis for the t(X;18) translocation was performed.
Figure 1.
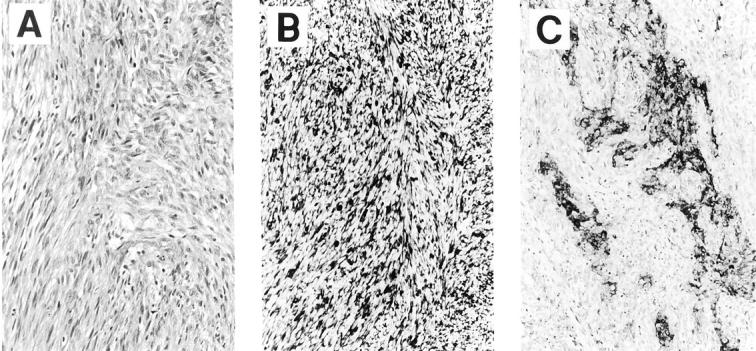
The biopsy of the soft tissue mass showed broad sweeping fascicles of spindle cells (A: H&E stain; magnification, ×50). The vimentin immunostain showed strong diffuse reactivity (B: magnification, ×50); the EMA immunostain highlighted the tumor’s focal epithelial component that had a vague glandular pattern (C: magnification, ×40).
Molecular Studies
The translocation t(X;18)(p11.2;q11.2) that is characteristic of synovial sarcoma has been consistently demonstrated in both monophasic and biphasic SS. 1 The translocation results in the fusion of the SYT gene located on chromosome 18 with one of three closely related SSX genes located on the X chromosome, and the predicted protein encoded by chimeric SYT-SSX fusion transcripts is composed of the N-terminal region of SYT fused to the C-terminal region of SSX1, SSX2, or SSX4. 1, 2, 3, 4 Although the biological properties of SYT and SSX proteins are largely unknown, SYT-SSX chimeric proteins are thought to result in an altered transcriptional pattern of specific, but as yet unknown, target genes. 1 Reverse transcriptase-polymerase chain reaction (RT-PCR) has been widely used to demonstrate the presence of SYT-SSX fusion transcripts in fresh tumor tissue as well as formalin-fixed, paraffin-embedded tissue. When there is adequate tissue for analysis, an SYT-SSX fusion transcript can be identified in over 90% of cases. 1, 2, 3, 5 Because of extensive homology between the SSX genes, RT-PCR can be performed using consensus primers that will amplify SYT-SSX fusion transcripts irrespective of the particular SSX gene involved, or using primer sets that permit identification of the specific SSX gene involved in the translocation. 5, 6, 7, 8, 9
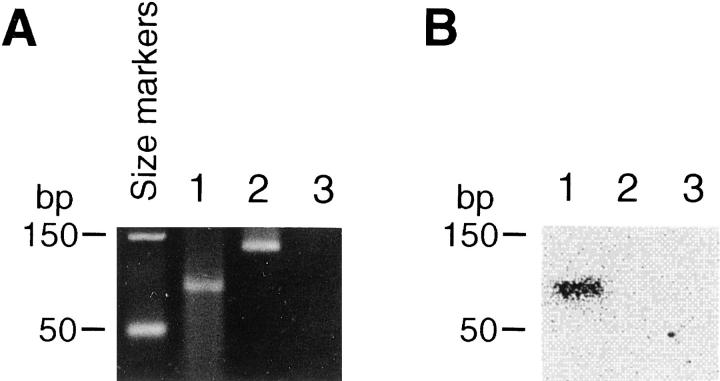
In the present case, RNA was extracted from the formalin-fixed, paraffin-embedded tissue and reverse transcribed, and the results of a single round of PCR performed using consensus primers 6, 10 are shown in Figure 2A . Two percent agarose gel electrophoresis of the PCR products demonstrated the expected 87-bp product from the positive control reaction but showed an atypically sized product from the tumor sample; as expected, the no-RT control sample was negative. Southern blot hybridization using a [32P]-radiolabeled oligonucleotide probe specific for the SYT-SSX fusion junction 6, 10 verified the identity of the control band, but showed no hybridization to the tumor band (Figure 2B) .
Figure 2.
RT-PCR analysis for t(X;18). Ethidium bromide stained 2% agarose gel (A) and corresponding Southern blot (B). Lane 1, positive control (formalin-fixed tissue from a genetically characterized SS); lane 2, patient sample; lane 3, negative control (no-RT control in which an aliquot from a cDNA synthesis reaction performed on the patient sample without added RT enzyme was subjected to PCR). The position of the DNA size markers is indicated.
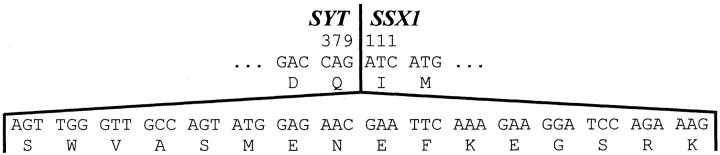
As is standard practice for all RT-PCR products generated in our molecular diagnostic laboratory, the PCR product was subcloned and its DNA sequence then determined, 10 which showed an SYT-SSX fusion with an in-frame 48-bp insert between the usual fusion boundaries (Figure 3) . A BLAST homology search (www.ncbi. nlm.nih.gov/BLAST/) indicated that the insert represents a so-called cryptic exon 11 derived from intron 4 of the SSX1 gene. Repeat analysis (using RNA extracted from additional sections of tumor tissue, followed by a single round of PCR using primers that discriminate between SYT-SSX1 and SYT-SSX2, 7 with subsequent DNA sequence analysis) confirmed the presence of the 48-bp insert between the usual fusion boundaries of an SYT-SSX1 chimeric transcript.
Figure 3.
DNA sequence of the 48-bp insert in the variant SYT-SSX fusion transcript. The predicted amino acid sequence is also shown. As indicated by the vertical line, the SYT derived portion of the fusion transcript ends with codon 379, and the portion derived from SSX1 begins with codon 111.
Discussion
For the vast majority of cases of SS that have been analyzed by molecular genetic methods, the junction of SYT and SSX in fusion transcripts occurs between codon 379 of SYT and codon 111 of SSX. However, the occurrence of rare variant SYT-SSX fusion transcripts is well established; in these cases, heterogeneity in the position of the breakpoint, coupled with variously sized inserts, produces atypically sized PCR products. 2, 3, 8, 12, 13, 14 Consequently, a band’s size based on gel electrophoresis alone can be an unreliable guide to its identity. Furthermore, evaluation of PCR products of an atypical size by Southern blot hybridization can be misleading, as this case demonstrates (because the variant transcript in the present case is unique, even an extensive library of probes corresponding to the known variant fusion transcripts would, in all likelihood, have been insufficient for correct classification by Southern blotting). Without foreknowledge of the identity of this variant transcript, only DNA sequence analysis provided unequivocal identification.
It is important to emphasize that the occurrence of variant or atypical fusion transcripts is not unique to SS, but has been described in several other sarcomas that are also associated with characteristic translocations. For example, variant EWS-FLI1 fusion transcripts in Ewing sarcoma/primitive neuroectodermal tumor (EWS/PNET) have been described that show cryptic exon inserts, 11 adding to the underlying complexity that is already present due to combinatorial joining of different exons of the EWS and FLI1 genes. 11 Similarly, a small insert has been reported in a variant chimeric EWS-WT1 transcript from a desmoplastic small round cell tumor. 15 Although it seems prudent to confirm the identity of any atypically sized PCR product by DNA sequence analysis, conventional cytogenetics and/or fluorescence in situ hybridization (FISH) may also be useful for verifying the presence of the related translocation.
Finally, it should be noted that fusion transcript type in SS has clinical significance in that an SYT-SSX2 chimeric transcript is associated with better overall survival. 2, 16 However, the prognostic implications of an atypical SYT-SSX fusion are unknown.
Address reprint requests to John D. Pfeifer, M.D., Ph.D., Department of Pathology and Immunology, Washington University School of Medicine, Campus Box 8118, 660 S. Euclid Ave., St. Louis, MO 63110-1093. E-mail: pfeifer@path.wustl.edu.
Footnotes
Maureen J. O’Sullivan’s current address is the Department of Pathology, Edinburgh University Medical School, Edinburgh, Scotland.
References
- 1.dos Santos NR, de Bruijn DRH, van Kessel AG: Molecular mechanisms underlying human synovial sarcoma development. Genes Chromosomes Cancer 2001, 30:1-14 [DOI] [PubMed] [Google Scholar]
- 2.Nilsson G, Skytting B, Xie Y, Brodin B, Perfekt R, Mandahl N, Lundeberg J, Uhlen M, Larsson O: The SYT-SSX1 variant of synovial sarcoma is associated with a high rate of tumor cell proliferation and poor clinical outcome. Cancer Res 1999, 59:3180-3184 [PubMed] [Google Scholar]
- 3.Crew AJ, Clark J, Fisher C, Gill S, Grimer R, Chand A, Shipley J, Gusterson BA, Cooper CS: Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to the Kruppel-associated box in human synovial sarcoma. EMBO J 1995, 14:2333-2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skytting B, Nilsson G, Brodin B, Xie Y, Lundeberg J, Uhlen M, Larsson O: A novel fusion gene SYT-SSX4, in synovial sarcoma. J Natl Cancer Inst 1999, 91:974-975 [DOI] [PubMed] [Google Scholar]
- 5.Lasota J, Jasinski M, Debiec-Rychter M, Szadowska A, Limon J, Miettinen M: Detection of the SYT-SSX fusion transcripts in formaldehyde-fixed, paraffin-embedded tissue: a reverse transcription-polymerase chain reaction amplification assay useful in the diagnosis of synovial sarcoma. Mod Pathol 1998, 11:626-633 [PubMed] [Google Scholar]
- 6.Argani P, Zakowski MF, Klimstra DS, Rosai J, Ladanyi M: Detection of the SYT-SSX chimeric RNA of synovial sarcoma in paraffin-embedded tissue and its application to problematic cases. Mod Pathol 1998, 11:65-71 [PubMed] [Google Scholar]
- 7.Tsuji S, Hisaoka M, Morimitsu Y, Hashimoto H, Shimajiri S, Komiya S, Ushijima M, Nakamura T: Detection of SYT-SSX fusion transcripts in synovial sarcoma by reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Am J Pathol 1998, 153:1807-1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Leeuw B, Balemans M, Weghuis DO, van Kessel AG: Identification of two alternative fusion genes, SYT-SSX1 and SYT-SSX2, in t(X;18)(p112;q112)-positive synovial sarcomas. Hum Mol Genet 1995, 4:1097-1099 [DOI] [PubMed] [Google Scholar]
- 9.Antonescu CR, Kawai A, Leung DH, Lonardo F, Woodruff JM, Healey JH, Ladanyi M: Strong association of SYT-SSX fusion type and morphologic epithelial differentiation in synovial sarcoma. Diagn Mol Pathol 2000, 9:1-8 [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan MJ, Kyriakos M, Zhu X, Wick MR, Swanson PE, Dehner LP, Humphrey PA, Pfeifer JD: Malignant peripheral nerve sheath tumors with t(X;18): a pathologic and molecular genetic study. Mod Pathol 2000, 13:1336-1346 [DOI] [PubMed] [Google Scholar]
- 11.Zucman J, Melot T, Desmaze C, Ghysdael J, Plougastel B, Peter M, Zucker JM, Triche TJ, Sheer D, Turc-Carel C, Ambros P, Combaret V, Lenoir G, Aurias A, Thomas G, Delattre O: Combinatorial generation of variable fusion proteins in the Ewing family of tumours. EMBO J 1993, 12:4481-4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fligman I, Lonardo F, Jhanwar SC, Gerald WL, Woodruff J, Ladanyi M: Molecular diagnosis of synovial sarcoma and characterization of a variant SYT-SSX2 fusion transcript. Am J Pathol 1995, 147:1592-1599 [PMC free article] [PubMed] [Google Scholar]
- 13.Safar A, Wickert R, Nelson M, Neff JR, Bridge JA: Characterization of a variant SYT-SSX1 synovial sarcoma fusion transcript. Diagn Mol Pathol 1998, 7:283-287 [DOI] [PubMed] [Google Scholar]
- 14.Sanders ME, van de Rijn M, Barr FG: Detection of a variant SYT-SSX1 fusion in a case of predominantly epithelioid synovial sarcoma. Mol Diagn 1999, 4:65-70 [DOI] [PubMed] [Google Scholar]
- 15.de Alava E, Ladanyi M, Rosai J, Gerald WL: Detection of chimeric transcripts in desmoplastic small round cell tumor and related developmental tumors by reverse transcriptase-polymerase chain reaction. Am J Pathol 1995, 147:1584-1591 [PMC free article] [PubMed] [Google Scholar]
- 16.Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, Healey JH, Brennan MF, Bridge JA, Neff JR, Barr FG, Goldsmith JD, Brooks JS, Goldblum JR, Ali SZ, Shipley J, Cooper CS, Fisher C, Skytting B, Larsson O: Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res 2002, 62:135-140 [PubMed] [Google Scholar]