Abstract
The compatible solute 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid (ectoine) acts in microorganisms as an osmotic counterweight against halostress and has attracted commercial attention as a protecting agent. Its production and application are restricted by the drawbacks of the discontinuous harvesting procedure involving salt shocks, which reduces volumetric yield, increases reactor corrosion, and complicates downstream processing. In order to synthesize ectoine continuously in less-aggressive media, we introduced the ectoine genes ectABC of the halophilic bacterium Chromohalobacter salexigens into an Escherichia coli strain using the expression vector pASK-IBA7. Under the control of a tet promoter, the transgenic E. coli synthesized 6 g liter−1 ectoine with a space-time yield of 40 mg liter−1 h−1, with the vast majority of the ectoine being excreted.
Halophilic microorganisms live in highly saline environments. There are two major strategies of adaptation to these hostile conditions. Extreme halophiles such as Halobacterium salinarum accumulate salt in the cytosol to maintain the osmotic balance (the salt-in strategy). Other halophiles synthesize and accumulate small organic molecules as osmotic counterweights (the organic-osmolyte strategy). Unlike intracellular salt, the small organic compounds do not affect the metabolism of the organism and are thus called compatible solutes. The enzymes of organic-osmolyte strategists do not need to be haloadapted, allowing these organisms to cope with strong salinity fluctuations. Non-salt-tolerant bacteria like Escherichia coli are usually unable to synthesize large amounts of compatible solutes but may resist halostress to a certain extent by taking up and accumulating compatible solutes (8, 9, 12, 27).
Different classes of chemical compounds, including polyols, sugars, methylamines, and linear and cyclic amino acids and betaines, have been found to act as compatible solutes. Besides functioning as osmotic counterweights, compatible solutes were shown to protect biomolecules and whole cells against denaturation caused by heating, freezing, desiccation, or chemical agents (15, 19, 22). This property has attracted commercial attention. Compatible solutes can be used as chemical chaperones for protein folding, enhancers of PCR performance, cryoprotectants of microorganisms, cosmeceuticals, and pharmaceuticals (20, 32). A potentially promising future application could be the enhancement of drought tolerance or salt tolerance of transgenic plants (1, 37).
The best-investigated compatible solute, 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid (ectoine), is biotechnologically produced with the halophilic bacterium Halomonas elongata (34). The physiology and genetics of ectoine biosynthesis in this bacterium have been studied in detail (7, 23, 24, 25, 26, 29). The technical bioprocess for the synthesis of ectoine exploits the salt adaptation strategy of H. elongata and is called “bacterial milking” (34). When the medium has a high salt concentration, the bacterium synthesizes and accumulates ectoine. After an osmotic down-shock, the cells counteract bursting by the sudden ejection of the ectoine. The technical process involves the cyclic increase and decrease of the salt concentration for ectoine production and ectoine milking, respectively. The weaknesses of the process are its high demand for the stability of the bioreactor materials and the difficult downstream processing of the product due to the discontinuous production scheme and high concentrations of salt. These lead to a relatively high price for ectoine, preventing some potential applications as a protector molecule.
To reduce the salt requirement, attempts were made to introduce the ectoine genes of halophiles into a nonhalophilic bacterium (E. coli). This recombinant strain synthesizes ectoine as a result of modest salt stress (21). The accumulation of ectoine in nonhalophilic strains by IPTG (isopropyl-β-d-thiogalactopyranoside)-induced gene expression has also been reported (10, 11, 16, 17). However, the heterologous synthesis of ectoine occurred only at such a low level that ectoine was undetectable extracellularly (10, 11, 16, 17, 21). In contrast, ectoine produced at higher rates should be ejected to maintain the osmotic equilibrium in low-saline medium via unspecific mechanosensitive channels. Mechanosensitive channels are known to act instantly upon osmotic down-shocks. These channels are found in the cell membranes of most microorganisms and are well known to extrude different intracellular solutes, e.g., potassium, amino acids, saccharides, and polyols (3, 5, 30, 35). Our reasoning was thus that a more powerful externally induced expression system should modify E. coli in such a way that the strain synthesizes high levels of ectoine, which is continuously excreted into the medium to prevent cell bursting. The goal of our study was to engineer such a strain and to investigate its potential benefits for the continuous production of ectoine under low-salinity conditions.
MATERIALS AND METHODS
Strains and media.
E. coli DH5α [F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1], as the host strain for bacterial transformation and plasmid propagation, was cultivated in Luria-Bertani medium or in a defined medium (DM) composed of the following (concentrations in mg liter−1): (NH4)2SO4 (6,000), K2HPO4 (4,400); KH2PO4 (3,400), CaCl2·6H2O (90), MgCl2·6H2O (1,100), glucose (10,000), ZnCl (3.5), MnCl2 (0.46), CuCl2 (7), Na2MoO4·2H2O (4.2), FeCl2 (38). The antibiotic ampicillin (100 mg liter−1) was used to maintain the heterologous plasmid in the genetically modified strain.
Molecular biological methods.
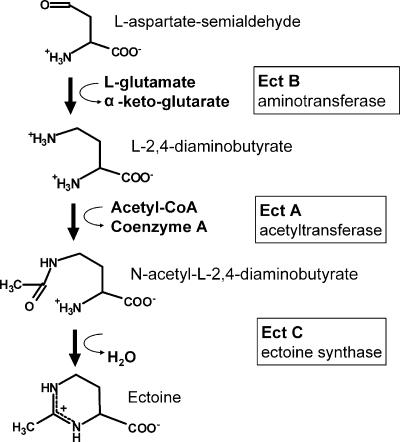
The ectABC gene cassette from Chromohalobacter salexigens DSM 3043 was used for the construction of the expression vector. ectA encodes diaminobutyrate acetylase, ectB encodes diaminobutyrate transaminase, and ectC encodes ectoine synthase (Fig. 1).
FIG. 1.
Biosynthesis of ectoine in Chromohalobacter salixigens. The genes ectB, ectA, and ectC encoding aminotransferase, acetyltransferase, and ectoine synthase, respectively, were introduced into E. coli.
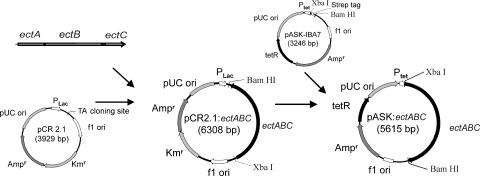
The ectABC gene cassette was amplified by PCR using the oligonucleotides 5′-ATG ACG CCT ACA ACC GAG AAC TTC A-3′ and 5′-TCA ATC GAC CGG TGC GTA-3′ and chromosomal DNA of C. salexigens as templates. The complete operon containing the genes ectA, ectB, and ectC was amplified without any terminator, operator, or promoter regions. The PCR fragments were cloned into cloning vector pCR2.1 by using the TA cloning kit (Invitrogen, Karlsruhe, Germany). The recombinant vector was transformed into E. coli DH5α. Plasmid DNA of the E. coli transformants was prepared as described by Birnboim and Doly (4). The BamHI-XbaI fragment from the recombinant cloning vector containing the ectABC gene cassette was recloned in the expression plasmid pASK-IBA7 (Fig. 2). The gene cassette was inserted downstream of the tet promoter. The resulting vector, pASK-ectABC, was transformed in E. coli DH5α. The transformation of E. coli followed the procedure described by Hanahan (13).
FIG. 2.
Design of the expression vector pASK-ectABC. The ectABC gene cassette from Chromohalobacter salexigens was initially inserted into pCR2.1 by PCR cloning using the TA cloning kit (Invitrogen) and then subcloned into the expression vector pASK-IBA7 with Ampr as the ampicillin resistance gene and tetR as the repressor gene of the tet promoter Ptet.
Bioreactor cultivation.
Precultures of E. coli DH5α(pASK-ectABC) were prepared in shaking flasks (30°C, 120 rpm) with DM containing ampicillin. The bioreactor was inoculated with exponentially growing bacteria. Batch cultivations were performed in a Biostat MD bioreactor (Sartorius BBI System GmbH, Melsungen, Germany) with a 1.5-liter working volume at 30°C and a pH of 7.0 ± 0.05, maintained by the automated addition of either 2.7 M NH4OH or 0.1 M H2SO4 as appropriate. The stirrer speed was 1,000 rpm and the aeration rate 3.0 liters min−1 (at a standard ambient temperature of 298.15 K and a standard ambient pressure of 105 Pa).
After the culture had reached a biomass concentration of 20 g liter−1, anhydrotetracycline was added (final concentration, 2 mg liter−1) to induce the expression of the ectoine genes. Glucose was added periodically after depletion.
Analyses.
The biomass was measured spectrophotometrically at 700 nm after calibration to bacterial dry mass. The concentration of glucose was measured by high-performance liquid chromatography (HPLC) using a Nucleosil carbohydrate column (Nucleogel 300 OA; Macherey-Nagel, Düren, Germany) with 0.01 N sulfuric acid in an isocratic eluent at 70°C (flow rate, 0.6 ml min−1). The refraction index detection system was used. To determine the ectoine concentration, isocratic HPLC using an NH2 column (Nucleosil 100-5 NH2; Macherey-Nagel) at 70°C with an acetonitrile-water (85%, vol/vol) solution as the mobile phase at a flow rate of 2.0 ml min−1 was applied. UV detection at 225 nm was used. Amino acids as well as a few other compatible solutes were quantified, and the ectoine measurements were confirmed by an HPLC analysis with pulsed amperometric detection as described by Riis et al. (31). The protein concentrations of the supernatant were measured by the method of Bradford (6), using bovine serum albumin as the standard.
Protein separation and identification.
Cell extracts of E. coli were prepared as described previously (2). Fifty micrograms of acetone-precipitated protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (18). Gels were stained with colloidal Coomassie brilliant blue and dried in a stream of unheated air. For mass spectrometric protein identification, bands of interest were excised, digested in gels with trypsin, and prepared for mass spectrometry (MS) (33). The samples were analyzed using an atmospheric pressure (AP) MALDI/TRAP-XCT mass spectrometer (Agilent Technologies, Palo Alto, CA) in automatic tandem MS (MS-MS) mode. The resulting MS-MS data were used for a database search with Mascot (Matrix Science) (28) against the NCBI database.
The assay for the acylation activity of EctA uses a coupled spectrometric test at 412 nm and contains 0.3 mM Ellman's reagent [5,5′-dithiobis (2-nitrobenzoic acid)] in 60 mM Tris-HCl (pH 8.5), 0.4 mM NaCl, 2 mM acetyl-coenzyme A, and 30 mM diaminobutyrate.
RESULTS AND DISCUSSION
Expression of the ectoine genes in E. coli and product synthesis.
The expression vector pASK-IBA7 carrying the ectABC gene cassette of C. salexigens downstream of the inducible promoter (Fig. 2) was transformed in E. coli DH5α. To verify the expression of the ectoine genes in the transgenic E. coli, they were induced with anhydrotetracycline and the activity of EctA was measured. The catalytic activity of EctA in lysates of the induced cells was 8 mU mg−1, in contrast to 2 mU mg−1 in lysates of noninduced cells. Due to the lack of appropriate assays, the specific activities of EctB and EctC could not be measured.
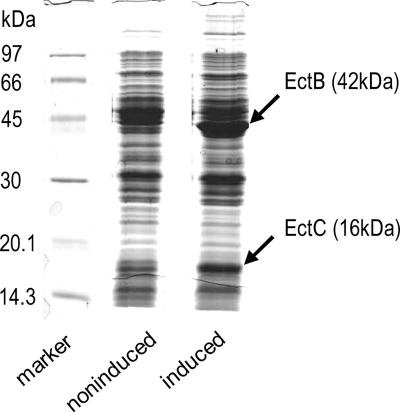
In order to compare the expression levels of ectB and ectC genes, extracts of induced and noninduced E. coli DH5α cells were used to visualize the respective proteins by SDS-PAGE. Lysates of induced cells showed two clearly amplified bands with molecular masses (42 kDa and 16 kDa) similar to those published for EctB and EctC (44 kDa and 19 kDa, respectively) (26). MS of tryptic digests of the 42-kDa band and the 16-kDa band identified EctB of Chromohalobacter salexigens DSM 3034 (five peptides, 20% sequence coverage; gi|67676419) and EctC of C. salexigens DSM 3034 (six peptides, 65% sequence coverage; gi|67519532), respectively. In each case, C. salexigens best matched the obtained peptide sequence in database searches. The heterologous expression of the ectoine genes ectB and ectC in E. coli was thus successful, and protein synthesis was amplified upon gene induction.
Due to the very similar masses, it is possible that EctA (19 kDa) comigrates with EctC in the 19-kDa band, and so no peptide matching EctA was detected by MS analysis. It is conceivable that the instability of EctA, already reported by Ono et al. (26), was responsible for the failure to identify EctA in the protein band containing EctC (Fig. 3).
FIG. 3.
SDS-PAGE of noninduced and induced cells of E. coli(pASK-ectABC). Arrows mark amplified bands after 24 h of induction. After the amplified bands were analyzed by MS, the bands were identified by a data bank search as the EctB and EctC proteins of C. salexigens.
The successful expression of ectABC in E. coli and the catalytic function of the three ectoine enzymes were also confirmed by the detection of ectoine by two different HPLC detection methods.
Growth and ectoine synthesis in recombinant E. coli.
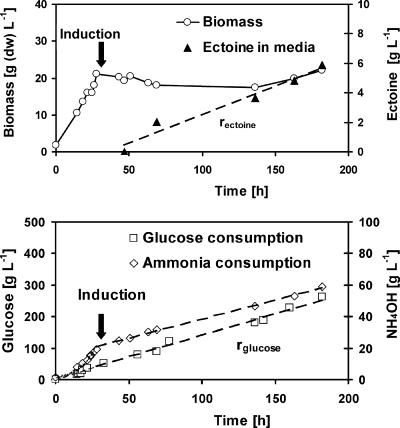
The use of an inducible promoter permitted the separation of an initial phase of biocatalyst production from a subsequent phase of ectoine synthesis. In the absence of the inducer in the first phase, the bacterium grew exponentially at a rate of 0.09 h−1 up to a biomass concentration of 22 g liter−1 (Fig. 4). No ectoine was detected during this phase. The second phase was initiated by adding anhydrotetracyline as an inducer. Growth ceased immediately, and ectoine was synthesized and excreted at a rate of 40 mg liter−1 h−1. After an induction time of 160 h, the concentrations of ectoine and biomass were 6.0 and 22 g liter−1, respectively. There was no indication of declining ectoine excretion up to this time. The ratio of extracellular ectoine to biomass of 0.27 exceeded the maximum ratio of intracellular ectoine to biomass of 0.2 obtained by halophilic strains, e.g., H. elongata (23), at this time. The specific ectoine synthesis rate of our recombinant E. coli strain (2 mg g−1 h−1) was of the same magnitude as the rate of the production strain H. elongata (7.1 mg g−1 h−1) (34). The cellular concentrations of ectoine during expression and overproduction were both 5 mg per g (dry weight).
FIG. 4.
Cultivation and ectoine synthesis in E. coli (pASK-ectABC) in a bioreactor (1.5-liter working volume) with DM at 30°C and pH 7.0. After 22 h (marked by an arrow), anhydrotetracycline was added to induce the expression of the ectoine genes. Metabolic fluxes in the induction phase are determined for ectoine synthesis (rectoine = 0.040 g liter−1 h−1) and for the corresponding glucose consumption (rglucose = 1.4 g liter−1 h−1) (curves are approximated to a linear rate).
Mechanism of ectoine excretion.
As the heterologous ectoine synthesis has no underlying physiological control, the risks of rising internal osmotic pressure and bursting of the bacterial cells exist. Bursting would lead to the discharge of intracellular protein and thus to an increase in the extracellular protein concentration. Before induction of ectoine synthesis, 0.4% (wt/wt) of the total protein (assuming 55% protein content of the biomass [36]) was found extracellularly, in contrast to the 5 to 7% extracellular protein found during the ectoine synthesis after induction with anhydrotetracyline. Thus, it seems that little protein was released while ectoine was excreted. The excretion of ectoine into the medium could have occurred via the well-investigated unspecific mechanosensitive channels (35) or specific transporters. In the first case, other amino acids should have been extruded as well, since E. coli, for instance, contains roughly 100 nmol glutamate per mg cell (dry weight) (35). This means that the glutamate concentration after activation of the mechanosensitive channels could have increased to 2,000 μM. This is far above the sensitivity level of our HPLC method (5 μM). Since we found no significant amounts of amino acids in the medium, we suppose that ectoine had been released by a specific efflux system. Osmoregulated secondary transporters for uptake of the compatible proline, glycine, betaine, and ectoine from the surrounding media are found in E. coli (14). Our own experiments showed that E. coli DH5α is able to take up ectoine when the salinity of the medium increases. This transport is independent from the genetic modifications as well as from the cultivation conditions (Fig. 5). Furthermore, Poolman and Glaasker (30) have described that such an osmotransporter can also function unidirectionally when activated by a slight increase in turgor pressure. It is therefore conceivable that the recombinant E. coli discharged ectoine by a specific transporter.
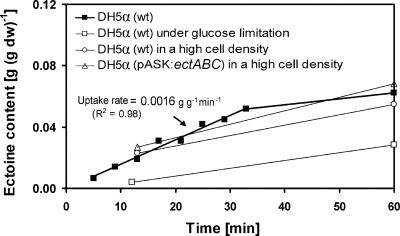
FIG. 5.
Uptake of exogenous ectoine (1 mM) from the medium into the cells of E. coli DH5α or noninduced E. coli(pASK-ectABC) under salt stress (final concentration, 4% NaCl). Intracellular ectoine was not found in the absence of exogenous ectoine source under salt stress. Without salt stress, only small intracellular amounts (ca. 0.1%) were detectable. The uptake rate of exogenous ectoine was 1.6 mg g−1 (dry weight [dw]) min−1. wt, wild type.
Furthermore, the DNA sequence between the tet promoter and the ectA start codon was analyzed as a 50-bp fragment with the sequence 5′- TTT GTA GCA CAA AGC TGA AAT GAA TAG TTC GAC AAA CAT CTA GCA TGC AT-3′. No additional functional genes that could be involved in the transport of ectoine were found.
Potential for further improvement of the biocatalyst.
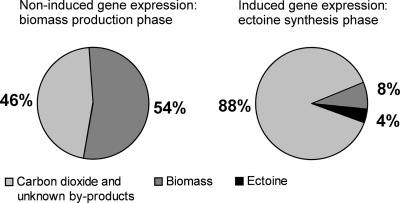
Figure 4 shows that the growth and production phases can be well controlled by using an anhydrotetracycline promoter. During the growth phase, 54% of the substrate carbon (C-mol) flowed into the growth and multiplication phases (Fig. 6). Although the ectoine synthesis rate and the ectoine/biomass ratio are promising indicators for the biotechnological application of our recombinant strain, the channeling of 88% of the carbon substrate into carbon dioxide and unknown by-products opens up opportunities for further strain optimizations. The main goal should be the search for metabolic reasons for the high catabolic rate and the low ectoine yield coefficient. Furthermore, other promoters should be tested as substitutes for the antibiotic anhydrotetracycline, since other inducers could be cheaper and less risky in terms of the induction of resistances or the contamination of the product. Another target for strain improvement is the integration of the heterologous genes into the chromosome to improve their stability in the host organism.
FIG. 6.
Carbon balance of the conversion of glucose in moles of C converted into biomass (growth), ectoine, and known by-products (e.g., CO2 [not measured]) under induced and noninduced conditions.
We have described here for the first time an engineered E. coli strain carrying the ectoine genes of the halophilic bacterium Chromohalobacter salexigens. The recombinant E. coli strain is promising for industrial application because it produces ectoine at high rates and excretes the product into the medium so that it can be easily separated from the biocatalyst. The observed low biomass formation during ectoine synthesis is rather advantageous for a potential bioprocess with immobilized cells. The established bioproduction of ectoine relies on cell recovery by cross-flow filtration that bears the risk of blockage. However, the main weaknesses of the conventional bioprocess, i.e., the complex downstream processing with extreme fluctuations in salinity and the resulting corrosiveness of the medium, can be overcome with our strain.
Acknowledgments
The AiF (German Federation of Industrial Research Associations Otto von Guericke) (KF 0218803 KMD3) is gratefully acknowledged for its financial support.
We thank Elke Häusler for her skilled technical assistance.
Footnotes
Published ahead of print on 16 March 2007.
REFERENCES
- 1.Apse, M. P., and E. Blumwald. 2002. Engineering salt tolerance in plants. Curr. Opin. Biotechnol. 3:146-150. [DOI] [PubMed] [Google Scholar]
- 2.Benndorf, D., I. Davidson, and W. Babel. 2004. Regulation of catabolic enzymes during long-term exposure of Delftia acidovorans MC1 to chlorophenoxy herbicides. Microbiology 150:1005-1014. [DOI] [PubMed] [Google Scholar]
- 3.Berrier, C., M. Besnard, B. Ajouz, A. Coulombe, and A. Ghazi. 1996. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J. Membr. Biol. 151:175-187. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blount, P., S. I. Sukharev, P. C. Moe, S. K. Nagle, and C. Kung. 1996. Towards an understanding of the structural and functional properties of MscL, a mechanosensitive channel in bacteria. Biol. Cell 87:1-8. [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Cánovas, D., C. Vargas, M. I. Calderon, A. Ventosa, and J. J. Nieto. 1998. Characterization of the genes for the biosynthesis of the compatible solute ectoine in the moderately halophilic bacterium Halomonas elongata DSM 3043. Syst. Appl. Microbiol. 21:487-497. [DOI] [PubMed] [Google Scholar]
- 8.Csonka, L. N. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53:121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galinski, E. A. 1995. Osmoadaptation in bacteria. Adv. Microb. Physiol. 37:272-328. [PubMed] [Google Scholar]
- 10.Galinski, E. A., and P. Louis. 1997. Verfahren zur Übertragung der Fähigkeit zur Biosynthese kompatibler Solute und der damit verbundenen Streβtoleranz auf Organismen. German patent DE 19610972 A1.
- 11.Galinski, E. A., T. Bestvater, P. Louis, and L. Eggeling. 1999. Verfahren zur Produktion von Ectoinen (1,4,5,6-tetrahydro-2-methyl-4-pyrimidincarbonsäure und 1,4,5,6-tetrahydro-2-methyl-5-hydroxy-4-pyrimidincarbonsäure) in nicht-halophilen Organismen unter Verwendung salzarmer Medien. German patent DE 199 25 615 A1.
- 12.Gustafsson, L., and B. Norkrans. 1976. On the mechanism of salt tolerance. Arch. Microbiol. 110:177-183. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 14.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 15.Knapp, S., R. Ladenstein, and E. A. Galinski. 1999. Extrinsic protein stabilisation by the naturally occurring osmolytes β-hydroxyectoine and betaine. Extremophiles. 3:191-198. [DOI] [PubMed] [Google Scholar]
- 16.Kuhlmann, A. U., and E. Bremer. 2002. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl. Environ. Microbiol. 68:772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunte, J., E. A. Galinski, T. Bestvater, A. Volke, and K. Grammann. 2001. Verfahren zur Gewinnung von Wertstoffen aus Organismen durch Beeinflussung/Beeinträchtigung der zelleigenen Transportsysteme für diese Wertstoffe bzw. durch Verwendung von Produktionsstämmen, denen besagteTransportsysteme fehlen. German patent DE 101 14 189 A1.
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lamosa, P., A. Burke, R. Peist, R. Huber, M.-Y. Liu, G. Silva, C. Rodrigues-Pousada, J. LeGall, C. Maycock, and H. Santos. 2000. Thermostabilization of proteins by diglycerol phosphate, a new compatible solute from hyperthermophile Archeoglobus fulgidus. Appl. Environ. Microbiol. 66:1974-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lentzen, G., and T. Schwarz. 2006. Extremolytes: natural compounds from extremophiles for versatile applications. Appl. Microbiol. Biotechnol. 72:623-634. [DOI] [PubMed] [Google Scholar]
- 21.Louis, P., and E. A. Galinski. 1997. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 143:1141-1149. [DOI] [PubMed] [Google Scholar]
- 22.Margesin, R., and F. Schinner. 2001. Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73-83. [DOI] [PubMed] [Google Scholar]
- 23.Maskow, T., and W. Babel. 2001. Calorimetrically obtained information about the efficiency of ectoine synthesis from glucose in Halomonas elongata. Biochim. Biophys. Acta 1527:4-10. [DOI] [PubMed] [Google Scholar]
- 24.Maskow, T., and H. Harms. 2006. Real time insights into bioprocesses using calorimetry: state of the art and potential. Eng. Life Sci. 6:266-277. [Google Scholar]
- 25.Maskow, T., and S. Kleinsteuber. 2004. Carbon and energy fluxes during haloadaptation of Halomonas sp. EF11 growing on phenol. Extremophiles 8:133-141. [DOI] [PubMed] [Google Scholar]
- 26.Ono, H., K. Sawada, N. Khunajakr, T. Tao, M. Yamamoto, M. Hiramoto, A. Shinmyo, M. Takano, and Y. Murooka. 1999. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J. Bacteriol. 181:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oren, A. 2002. Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J. Ind. Microbiol. Biotechnol. 28:56-63. [DOI] [PubMed] [Google Scholar]
- 28.Perkins, D. N., D. J. Pappin, D. M. Creasy, and J. S. Cottrell. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551-3567. [DOI] [PubMed] [Google Scholar]
- 29.Peters, P., E. A. Galinski, and H. G. Trüper. 1990. The biosynthesis of ectoine. FEMS Microbiol. Lett. 71:157-162. [Google Scholar]
- 30.Poolman, B., and E. Glaasker. 1998. Regulation of compatible solute accumulation in bacteria. Mol. Microbiol. 29:397-407. [DOI] [PubMed] [Google Scholar]
- 31.Riis, V., T. Maskow, and W. Babel. 2003. Highly sensitive determination of ectoine and other compatible solutes by anion-exchange chromatography and pulsed amperometric detection. Anal. Bioanal. Chem. 377:203-207. [DOI] [PubMed] [Google Scholar]
- 32.Roberts, M. F. 2005. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Systems 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos, P. M., D. Benndorf, and I. Sa-Correia. 2004. Insights into Pseudomonas putida KT2440 response to phenol-induced stress by quantitative proteomics. Proteomics 4:2640-2652. [DOI] [PubMed] [Google Scholar]
- 34.Sauer, T., and E. A. Galinski. 1998. Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol. Bioeng. 57:306-313. [PubMed] [Google Scholar]
- 35.Schleyer, M., R. Schmid, and E. P. Bakker. 1993. Transient, specific and extremely rapid release of osmolytes from growing cells of Escherichia coli K-12 exposed to hypoosmotic shock. Arch. Microbiol. 160:424-431. [DOI] [PubMed] [Google Scholar]
- 36.Stephanopoulos, G. N., A. A. Aristidou, and J. Nielsen. 1998. Metabolic engineering, principles and methodologies, p. 22. Academic Press, San Diego, CA.
- 37.Yoshida, K. 2002. Plant biotechnology—genetic engineering to enhance plant salt tolerance. J. Biosci. Bioeng. 94:585-590. [DOI] [PubMed] [Google Scholar]