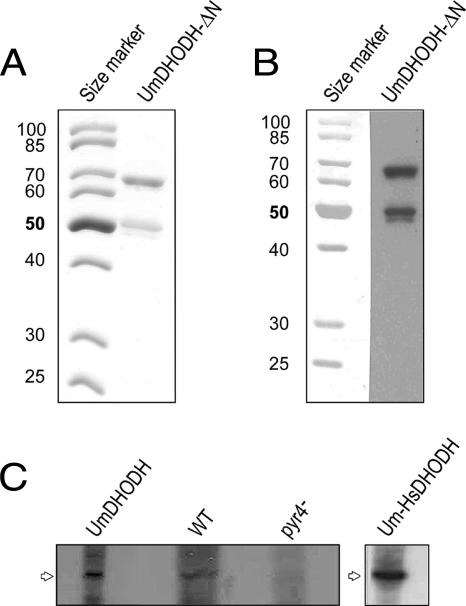
FIG. 2.
Expression of recombinant U. maydis dihydroorotate dehydrogenase in E. coli and Western analysis of mitochondrial proteins. (A) The N-terminally truncated U. maydis DHODH (UmDHODH-ΔN) was expressed in E. coli and purified by affinity chromatography. Shown is a Coomassie-stained SDS gel of the purified protein (2 μg). (B) Western blot of purified U. maydis DHODH-ΔN. The purified protein (1 μg) was separated by SDS-PAGE and immunostained with rabbit polyclonal anti-human DHODH serum. (C) Mitochondria were prepared from an FB1 Δpyr4 mutant strain transformed with U. maydis DHODH (UmDHODH), the wild-type (WT) FB1 strain, an FB1 Δpyr4 mutant strain (pyr4−), and an FB1 Δpyr4 mutant strain expressing the chimeric U. maydis-human DHODH construct (Um-HsDHODH); separated by SDS-PAGE; and immunostained with rabbit polyclonal anti-human DHODH serum.