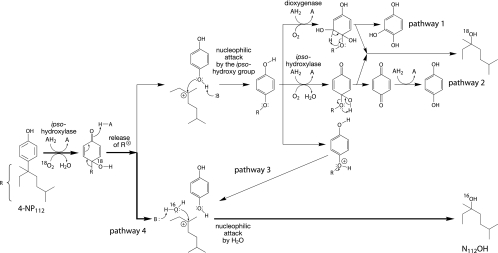
FIG. 1.
Potential pathways for the degradation of α-quaternary 4-nonylphenols in Sphingobium xenophagum Bayram. Nonylphenols are transformed by an initial ipso-hydroxylation to 4-alkyl-4-hydroxy-cyclohexadienone intermediates. α-Quaternary intermediates dissociate by releasing the alkyl moiety as a cation, which is stabilized by α-alkyl branching (14). Degradation experiments with 18O-labeled oxygen and water clearly show that the detached cation preferentially reacts with the nucleophilic oxygen atom of a water molecule (pathway 4). However, the degradation of a small fraction (up to 13%) of certain α,α-dimethyl-substituted nonylphenols appears to proceed on a minor pathway, in which the cation reacts with the ipso-hydroxy group (pathway 2, or possibly pathway 1). Pathway 3 was ruled out by showing that the ether oxygen in 4-t-butoxyphenol, a structural analogue of α-quaternary 4-nonyloxyphenols, was retained in the t-butanol metabolite. To show incorporation of molecular oxygen during the ipso-hydroxylation step, 4-(1-methyl-octyl)-phenol, which contains an α-hydrogen atom and is converted to accumulating ipso-hydroxylated metabolites, was used as a substrate analogue (13, 14). AH2 and A, a pair of reduced and oxidized cosubstrates, respectively; B, a proton acceptor.