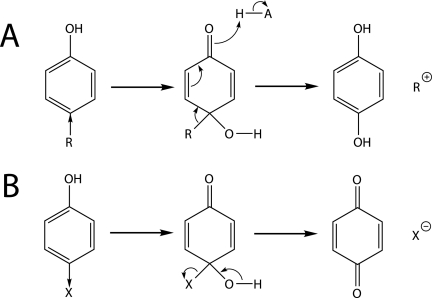
FIG. 7.
ipso-Substitution of phenolic compounds that have para-substituents with electron-donating (A) or electron-withdrawing (B) properties (as indicated by the direction of the arrow). The substituent is eliminated formally either as a cation (A) or as an anion (B). Depending on whether the ring-substituent bond electrons in the cyclohexadienone derivative remain with the carbon ring or the substituent, hydroquinone (A) or p-quinone (B) is formed (7, 14, 24, 30, 39). Protonation of alkoxy side chains transforms them into good leaving groups (Fig. 6).