Abstract
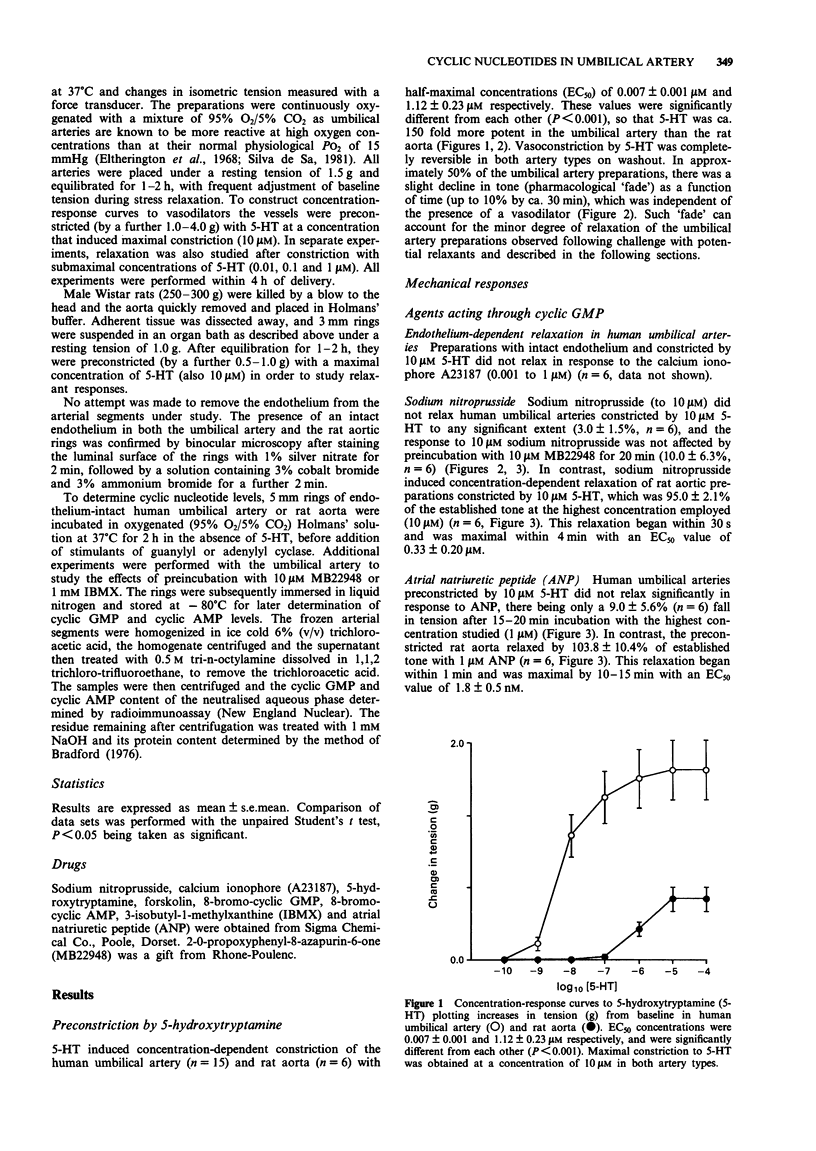
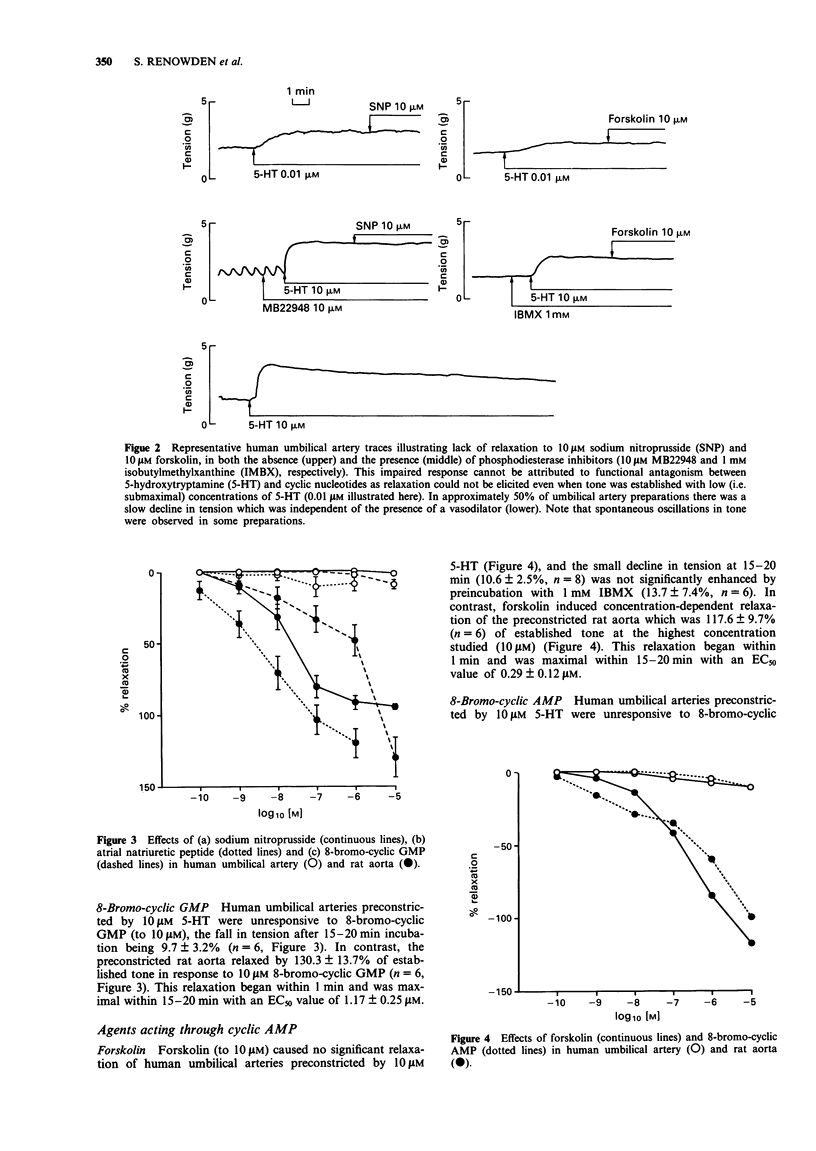
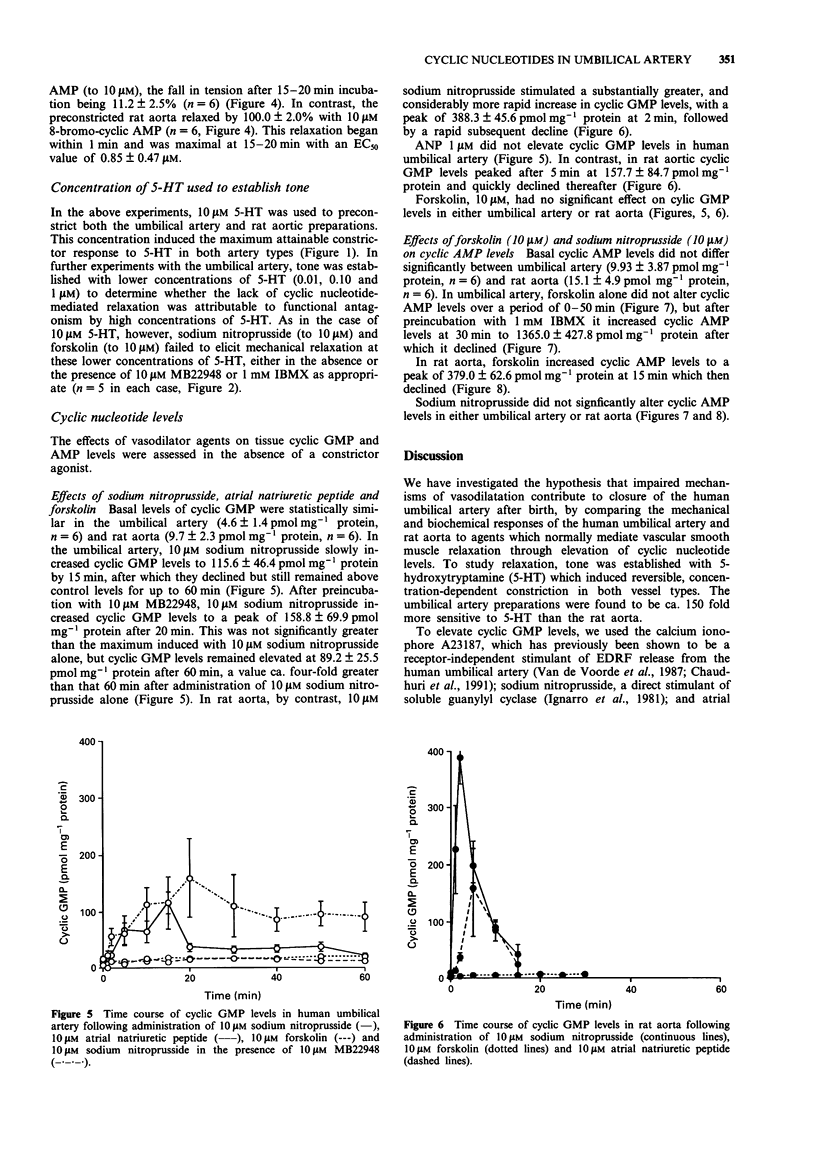
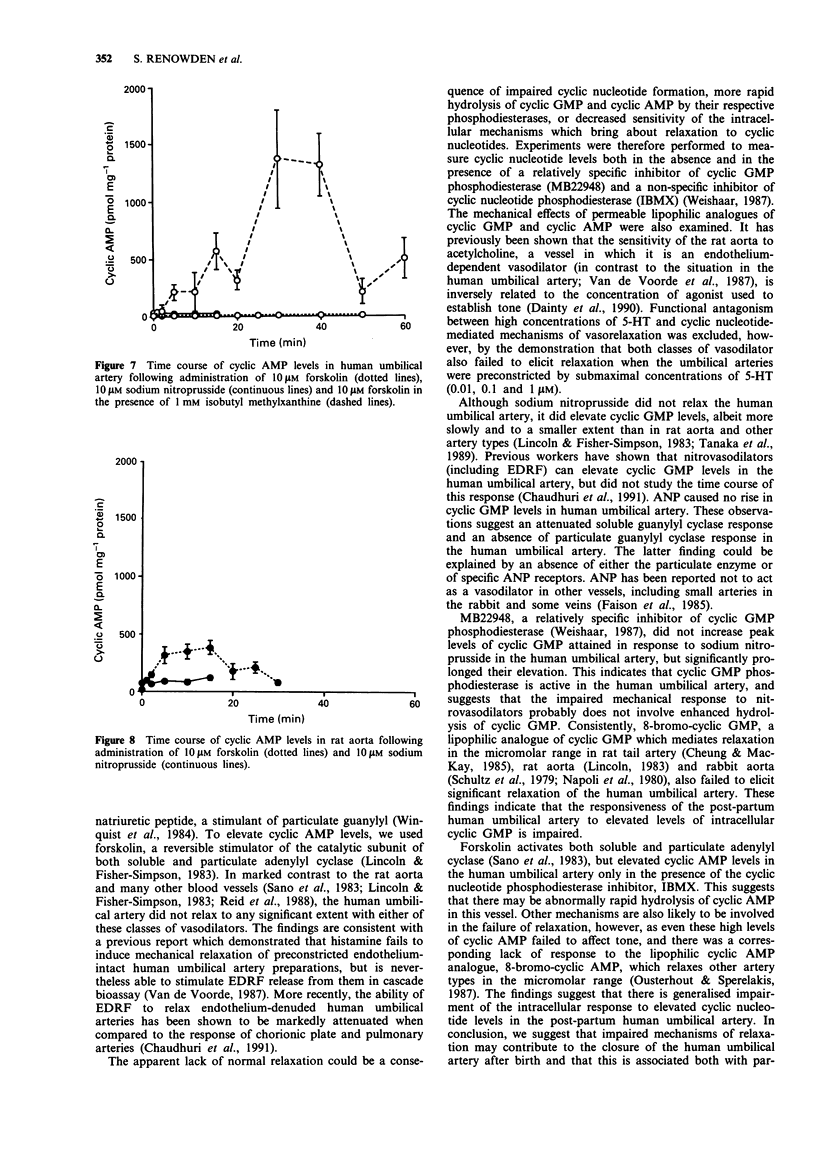
1. The mechanical and biochemical effects of agents that relax vascular smooth muscle either through elevation of guanosine 3':5'-cyclic monophosphate (cyclic GMP) or adenosine 3':5'-cyclic monophosphate (cyclic AMP) levels were compared in isolated ring preparations of human umbilical artery and rat aorta. Tone was established by preconstriction with 5-hydroxytryptamine. 2. The endothelium-dependent vasodilator calcium ionophore (A23187) (which stimulates endothelium-derived relaxing factor [EDRF] release and thus acts through soluble guanylyl cyclase), sodium nitroprusside (which stimulates soluble guanylyl cyclase directly), and atrial natriuretic peptide (which stimulates particulate guanylyl cyclase) relaxed rat aorta but not human umbilical artery. 3. Sodium nitroprusside, 10 microM, increased cyclic GMP levels from 10 to 390 pmol mg-1 protein at 2 min in rat aorta, as compared with a slower, relatively attenuated rise from 5 to 116 pmol mg-1 protein after 15 min in human umbilical artery. The rise in cyclic GMP in the umbilical artery was not significantly augmented by the cyclic GMP phosphodiesterase inhibitor, MB22948. Atrial natriuretic peptide increased cyclic GMP levels in rat aorta but not in human umbilical artery. 4. Forskolin, 10 microM, which stimulates both soluble and particulate adenylyl cyclase, maximally relaxed rat aorta and increased cyclic AMP levels from 15 to 379 pmol mg-1 protein at 15 min, but did not significantly relax or increase cyclic AMP levels in human umbilical artery. After preincubation with the cyclic nucleotide phosphodiesterase inhibitor, IBMX, 10 microM forskolin increased cyclic AMP levels to 1365 pmol mg-1 protein at 30 min in human umbilical arteries, but these high levels were not accompanied by mechanical relaxation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Malaviya D., Reich C. F., Orkin L. R. Effects of vasoactive agents on isolated human umbilical arteries and veins. Am J Physiol. 1972 Feb;222(2):345–355. doi: 10.1152/ajplegacy.1972.222.2.345. [DOI] [PubMed] [Google Scholar]
- BARTELS H., MOLL W., METCALFE J. Physiology of gas exchange in the human placenta. Am J Obstet Gynecol. 1962 Dec 1;84:1714–1730. doi: 10.1016/0002-9378(62)90012-1. [DOI] [PubMed] [Google Scholar]
- Bhalla R. C., Webb R. C., Singh D., Brock T. Role of cyclic AMP in rat aortic microsomal phosphorylation and calcium uptake. Am J Physiol. 1978 May;234(5):H508–H514. doi: 10.1152/ajpheart.1978.234.5.H508. [DOI] [PubMed] [Google Scholar]
- Bjøro K., Stray-Pedersen S. In vitro perfusion studies on human umbilical arteries. I. Vasoactive effects of serotonin, PGF2 alpha and PGE2. Acta Obstet Gynecol Scand. 1986;65(4):351–355. doi: 10.3109/00016348609157359. [DOI] [PubMed] [Google Scholar]
- Boura A. L., Boyle L., Sinnathuray T. A., Walters W. A. Release of prostaglandins during contraction of the human umbilical vein on reduction of temperature. Br J Pharmacol. 1979 Mar;65(3):360–362. doi: 10.1111/j.1476-5381.1979.tb07838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chaudhuri G., Buga G. M., Gold M. E., Wood K. S., Ignarro L. J. Characterization and actions of human umbilical endothelium derived relaxing factor. Br J Pharmacol. 1991 Feb;102(2):331–336. doi: 10.1111/j.1476-5381.1991.tb12174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W., MacKay M. J. The effects of sodium nitroprusside and 8-bromo-cyclic GMP on electrical and mechanical activities of the rat tail artery. Br J Pharmacol. 1985 Sep;86(1):117–124. doi: 10.1111/j.1476-5381.1985.tb09441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Griffith T. M., Henderson A. H., Lewis M. J. Endothelium-derived relaxing factor alters calcium fluxes in rabbit aorta: a cyclic guanosine monophosphate-mediated effect. J Physiol. 1986 Dec;381:427–437. doi: 10.1113/jphysiol.1986.sp016336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell T. L., Lincoln T. M. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem. 1989 Jan 15;264(2):1146–1155. [PubMed] [Google Scholar]
- Dainty I. A., McGrath J. C., Spedding M., Templeton A. G. The influence of the initial stretch and the agonist-induced tone on the effect of basal and stimulated release of EDRF. Br J Pharmacol. 1990 Aug;100(4):767–773. doi: 10.1111/j.1476-5381.1990.tb14090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltherington L. G., Stoff J., Hughes T., Melmon K. L. Constriction of human umbilical arteries. Interaction between oxygen and bradykinin. Circ Res. 1968 Jun;22(6):747–752. doi: 10.1161/01.res.22.6.747. [DOI] [PubMed] [Google Scholar]
- Faison E. P., Siegl P. K., Morgan G., Winquist R. J. Regional vasorelaxant selectivity of atrial natriuretic factor in isolated rabbit vessels. Life Sci. 1985 Sep 16;37(11):1073–1079. doi: 10.1016/0024-3205(85)90599-5. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Henderson A. H. Evidence that cyclic guanosine monophosphate (cGMP) mediates endothelium-dependent relaxation. Eur J Pharmacol. 1985 Jun 7;112(2):195–202. doi: 10.1016/0014-2999(85)90496-0. [DOI] [PubMed] [Google Scholar]
- Hirata M., Kohse K. P., Chang C. H., Ikebe T., Murad F. Mechanism of cyclic GMP inhibition of inositol phosphate formation in rat aorta segments and cultured bovine aortic smooth muscle cells. J Biol Chem. 1990 Jan 25;265(3):1268–1273. [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Karim S. M. The identification of prostaglandins in human umbilical cord. Br J Pharmacol Chemother. 1967 Feb;29(2):230–237. doi: 10.1111/j.1476-5381.1967.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T. M. Effects of nitroprusside and 8-bromo-cyclic GMP on the contractile activity of the rat aorta. J Pharmacol Exp Ther. 1983 Jan;224(1):100–107. [PubMed] [Google Scholar]
- Lincoln T. M., Fisher-Simpson V. A comparison of the effects of forskolin and nitroprusside on cyclic nucleotides and relaxation in the rat aorta. Eur J Pharmacol. 1984 May 18;101(1-2):17–27. doi: 10.1016/0014-2999(84)90026-8. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Cline M. J., Hughes T., Nies A. S. Kinins: possible mediators of neonatal circulatory changes in man. J Clin Invest. 1968 Jun;47(6):1295–1302. doi: 10.1172/JCI105821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli S. A., Gruetter C. A., Ignarro L. J., Kadowitz P. J. Relaxation of bovine coronary arterial smooth muscle by cyclic GMP, cyclic AMP and analogs. J Pharmacol Exp Ther. 1980 Mar;212(3):469–473. [PubMed] [Google Scholar]
- Ousterhout J. M., Sperelakis N. Cyclic nucleotides depress action potentials in cultured aortic smooth muscle cells. Eur J Pharmacol. 1987 Nov 24;144(1):7–14. doi: 10.1016/0014-2999(87)90003-3. [DOI] [PubMed] [Google Scholar]
- Popescu L. M., Panoiu C., Hinescu M., Nutu O. The mechanism of cGMP-induced relaxation in vascular smooth muscle. Eur J Pharmacol. 1985 Jan 8;107(3):393–394. doi: 10.1016/0014-2999(85)90269-9. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M. Cyclic guanosine monophosphate inhibition of contraction may be mediated through inhibition of phosphatidylinositol hydrolysis in rat aorta. Circ Res. 1986 Mar;58(3):407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- Reid D. L., Hollister M. C., Phernetton T. M., Rankin J. H. Effects of forskolin on placental vascular resistance in rabbits. Proc Soc Exp Biol Med. 1988 Sep;188(4):451–454. doi: 10.3181/00379727-188-42759. [DOI] [PubMed] [Google Scholar]
- Reilly F. D., Russell P. T. Neurohistochemical evidence supporting an absence of adrenergic and cholinergic innervation in the human placenta and umbilical cord. Anat Rec. 1977 Jul;188(3):277–286. doi: 10.1002/ar.1091880302. [DOI] [PubMed] [Google Scholar]
- Sano M., Kitajima S., Mizutani A. Activation of adenylate cyclase by forskolin in rat brain and testis. Arch Biochem Biophys. 1983 Feb 1;220(2):333–339. doi: 10.1016/0003-9861(83)90421-6. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Fay F. S. Transmembrane 45Ca fluxes in isolated smooth muscle cells: basal Ca2+ fluxes. Am J Physiol. 1984 May;246(5 Pt 1):C422–C430. doi: 10.1152/ajpcell.1984.246.5.C422. [DOI] [PubMed] [Google Scholar]
- Schultz K. D., Böhme E., Kreye V. A., Schultz G. Relaxation of hormonally stimulated smooth muscular tissues by the 8-bromo derivative of cyclic GMP. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jan;306(1):1–9. doi: 10.1007/BF00515586. [DOI] [PubMed] [Google Scholar]
- Silva de Sá M. F., Meirelles R. S., Franco J. G., Jr, Rodrigues R. Constriction of human umbilical artery induced by local anesthetics. Gynecol Obstet Invest. 1981;12(3):123–131. doi: 10.1159/000299594. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Kuriyama H. Effects of cAMP- and cGMP-dependent protein kinases, and calmodulin on Ca2+ uptake by highly purified sarcolemmal vesicles of vascular smooth muscle. Biochim Biophys Acta. 1984 Jun 13;773(1):83–90. doi: 10.1016/0005-2736(84)90552-2. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Muramatsu M., Aihara H. Alteration of cyclic GMP metabolism by CD-349, a novel calcium antagonist, and by sodium nitroprusside in bovine intrapulmonary artery and vein. Biochem Pharmacol. 1989 Jun 15;38(12):1985–1991. doi: 10.1016/0006-2952(89)90498-x. [DOI] [PubMed] [Google Scholar]
- Tuvemo T., Strandberg K., Hamberg M., Samuelsson B. Formation and action of prostaglandin endoperoxides in the isolated human umbilical artery. Acta Physiol Scand. 1976 Feb;96(2):145–149. doi: 10.1111/j.1748-1716.1976.tb10183.x. [DOI] [PubMed] [Google Scholar]
- Twort C. H., van Breemen C. Cyclic guanosine monophosphate-enhanced sequestration of Ca2+ by sarcoplasmic reticulum in vascular smooth muscle. Circ Res. 1988 May;62(5):961–964. doi: 10.1161/01.res.62.5.961. [DOI] [PubMed] [Google Scholar]
- Van de Voorde J., Vanderstichele H., Leusen I. Release of endothelium-derived relaxing factor from human umbilical vessels. Circ Res. 1987 Apr;60(4):517–522. doi: 10.1161/01.res.60.4.517. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E. Multiple molecular forms of phosphodiesterase: an overview. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(7):463–472. [PubMed] [Google Scholar]
- Winquist R. J., Faison E. P., Waldman S. A., Schwartz K., Murad F., Rapoport R. M. Atrial natriuretic factor elicits an endothelium-independent relaxation and activates particulate guanylate cyclase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7661–7664. doi: 10.1073/pnas.81.23.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]


