Abstract
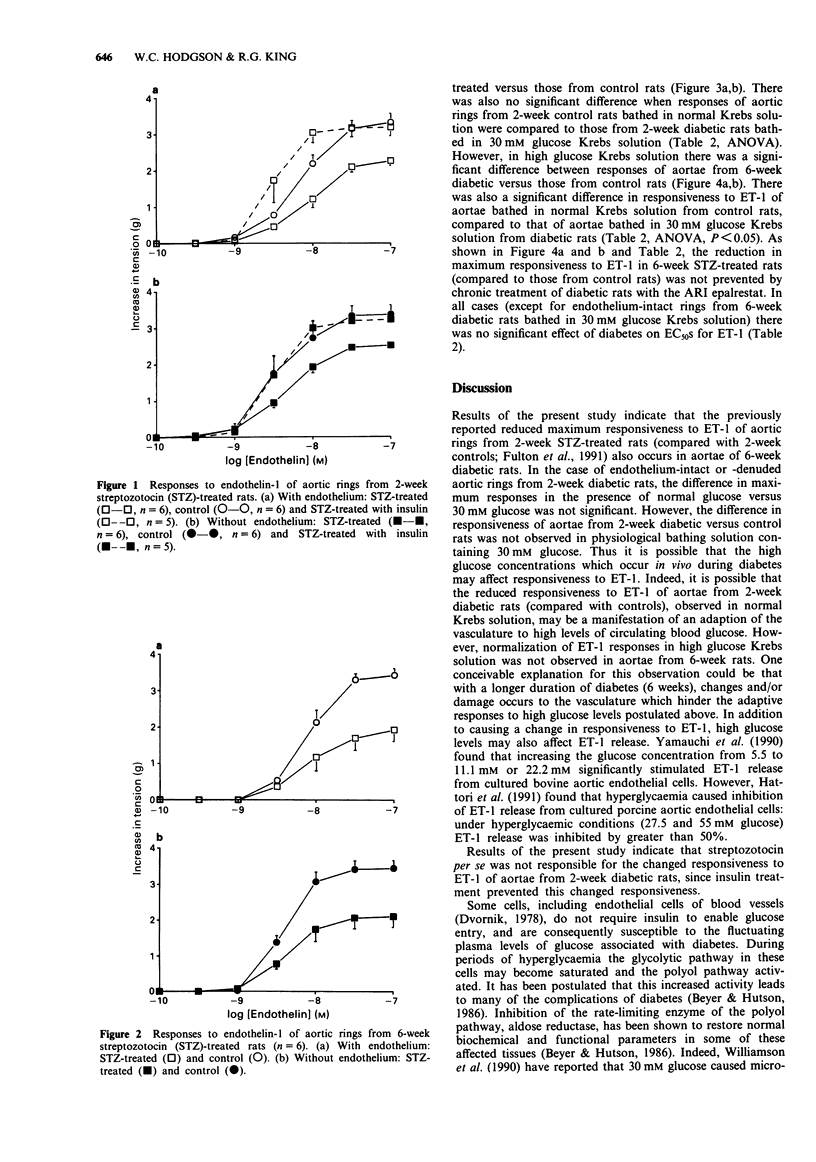
1. This study investigated the constrictor responsiveness to endothelin-1 (ET-1, 0.1 nM-0.1 microM) of aortic rings (under 10 g resting tension in Krebs solution) from 2- and 6-week streptozotocin (STZ, 60 mg kg-1, i.v.)-induced diabetic rats and vehicle-treated control rats. 2. In aortae from 2- and 6-week STZ-treated rats, and their corresponding controls, removal of endothelium caused leftward shifts of ET-1 concentration-response curves without affecting maximum responses. 3. Maximum responses to ET-1 were reduced in aortae from both 2- and 6-week STZ-treated rats compared to those from control rats. Such reductions were still evident after removal of the endothelium. 4. Decreased responsiveness to ET-1 of aortae from 2-week STZ-treated rats was still evident after chronic treatment with the aldose reductase inhibitor epalrestat, but not after chronic insulin treatment or in aortae bathed in high glucose (30 mM) Krebs solution. 5. Decreased responsiveness to ET-1 of aortae from 6-week STZ-treated rats (compared with those from controls) was still evident after chronic epalrestat treatment and in high glucose Krebs solution. 6. These data suggest that the decreased responsiveness to ET-1 observed in aortae from 2- and 6-week STZ-induced diabetic rats is not due to abnormal activity of the polyol pathway. The altered responsiveness in aortae from 2-week diabetic rats (compared with those from control rats) may possibly be a manifestation of changes (adaptive or otherwise) which occur as a result of high glucose concentrations in vivo.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
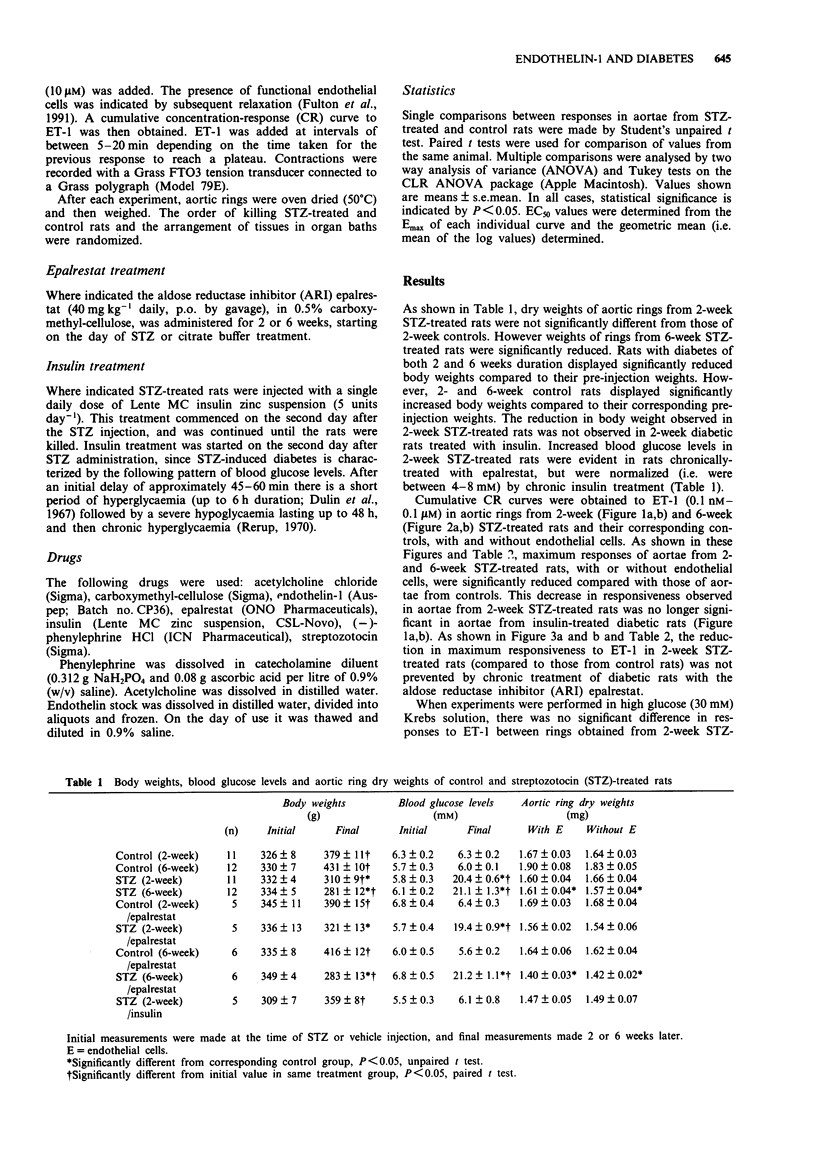
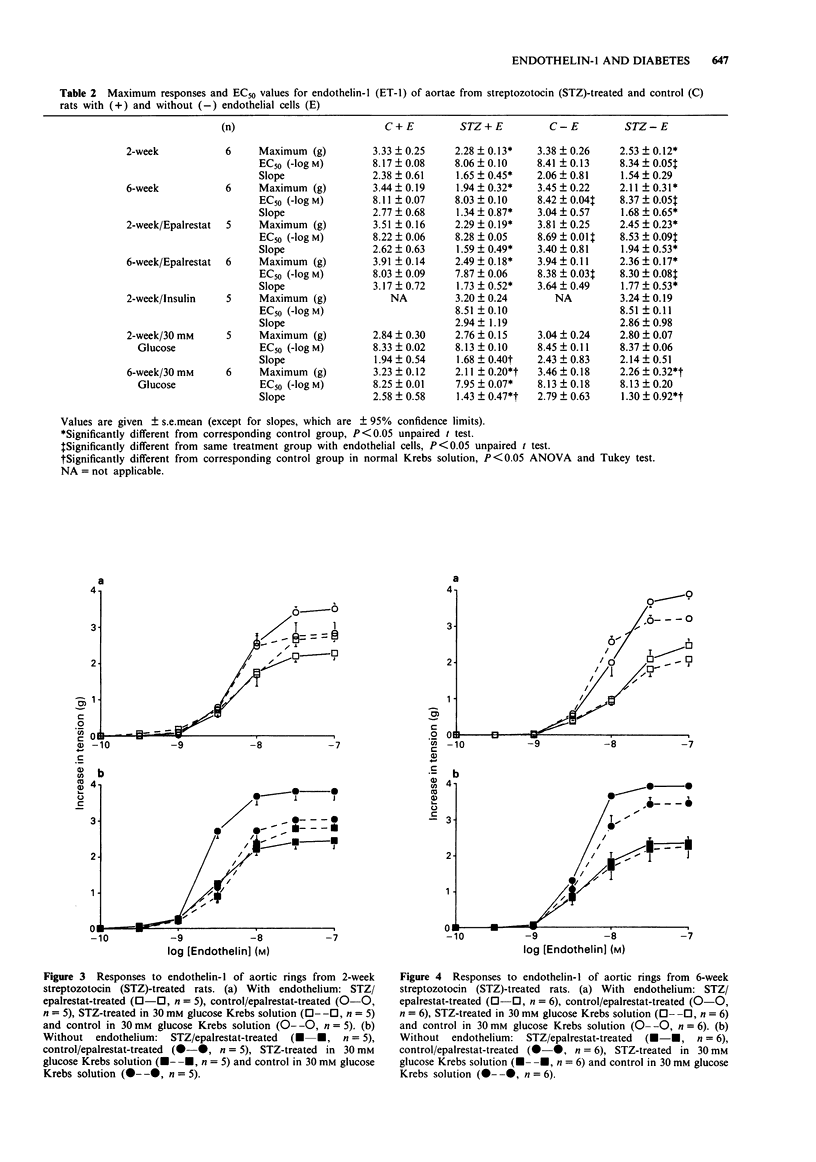


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer T. A., Hutson N. J. Introduction: evidence for the role of the polyol pathway in the pathophysiology of diabetic complications. Metabolism. 1986 Apr;35(4 Suppl 1):1–3. doi: 10.1016/0026-0495(86)90178-2. [DOI] [PubMed] [Google Scholar]
- Fulton D. J., Hodgson W. C., Sikorski B. W., King R. G. Attenuated responses to endothelin-1, KCl and CaCl2, but not noradrenaline, of aortae from rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1991 Dec;104(4):928–932. doi: 10.1111/j.1476-5381.1991.tb12528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Satoh N., Takiguchi Y., Nakashima M. Effects of an aldose reductase inhibitor, ONO-2235, insulin and their combination on the altered responsiveness to the nerve stimulation and agonists of the isolated atria of diabetic rats. J Pharmacol Exp Ther. 1990 May;253(2):552–557. [PubMed] [Google Scholar]
- Hattori Y., Kasai K., Nakamura T., Emoto T., Shimoda S. Effect of glucose and insulin on immunoreactive endothelin-1 release from cultured porcine aortic endothelial cells. Metabolism. 1991 Feb;40(2):165–169. doi: 10.1016/0026-0495(91)90168-v. [DOI] [PubMed] [Google Scholar]
- Head R. J., Longhurst P. A., Panek R. L., Stitzel R. E. A contrasting effect of the diabetic state upon the contractile responses of aortic preparations from the rat and rabbit. Br J Pharmacol. 1987 Jun;91(2):275–286. doi: 10.1111/j.1476-5381.1987.tb10282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa R., Hatanaka I., Yasuda H., Kobayashi N., Shigeta Y. Prevention of peripheral nerve dysfunction by an aldose reductase inhibitor in streptozotocin-diabetic rats. Metabolism. 1984 Mar;33(3):212–214. doi: 10.1016/0026-0495(84)90038-6. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Yang Z. H., Diederich D., Bühler F. R. Endothelium-derived vasoactive substances: potential role in hypertension, atherosclerosis, and vascular occlusion. J Cardiovasc Pharmacol. 1989;14 (Suppl 6):S63–S69. [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Liu J. J., Panagiotopoulos S., Casley D. J. Streptozotocin-induced diabetes reduces the density of [125I]-endothelin-binding sites in rat cardiac membranes. Br J Pharmacol. 1989 Aug;97(4):993–995. doi: 10.1111/j.1476-5381.1989.tb12552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Rodman D. M., McMurtry I. F., Peach J. L., O'Brien R. F. Comparative pharmacology of rat and porcine endothelin in rat aorta and pulmonary artery. Eur J Pharmacol. 1989 Jun 20;165(2-3):297–300. doi: 10.1016/0014-2999(89)90724-3. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Ansari N. H., Hair G. A., Awasthi S., Das B. Activation of human erythrocyte, brain, aorta, muscle, and ocular tissue aldose reductase. Metabolism. 1986 Apr;35(4 Suppl 1):114–118. doi: 10.1016/0026-0495(86)90199-x. [DOI] [PubMed] [Google Scholar]
- Topouzis S., Huggins J. P., Pelton J. T., Miller R. C. Modulation by endothelium of the responses induced by endothelin-1 and by some of its analogues in rat isolated aorta. Br J Pharmacol. 1991 Feb;102(2):545–549. doi: 10.1111/j.1476-5381.1991.tb12208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Ostrow E., Eades D., Chang K., Allison W., Kilo C., Sherman W. R. Glucose-induced microvascular functional changes in nondiabetic rats are stereospecific and are prevented by an aldose reductase inhibitor. J Clin Invest. 1990 Apr;85(4):1167–1172. doi: 10.1172/JCI114549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T., Ohnaka K., Takayanagi R., Umeda F., Nawata H. Enhanced secretion of endothelin-1 by elevated glucose levels from cultured bovine aortic endothelial cells. FEBS Lett. 1990 Jul 2;267(1):16–18. doi: 10.1016/0014-5793(90)80276-o. [DOI] [PubMed] [Google Scholar]
- Yorek M. A., Dunlap J. A. The effect of elevated glucose levels on myo-inositol metabolism in cultured bovine aortic endothelial cells. Metabolism. 1989 Jan;38(1):16–22. doi: 10.1016/0026-0495(89)90174-1. [DOI] [PubMed] [Google Scholar]


