Abstract
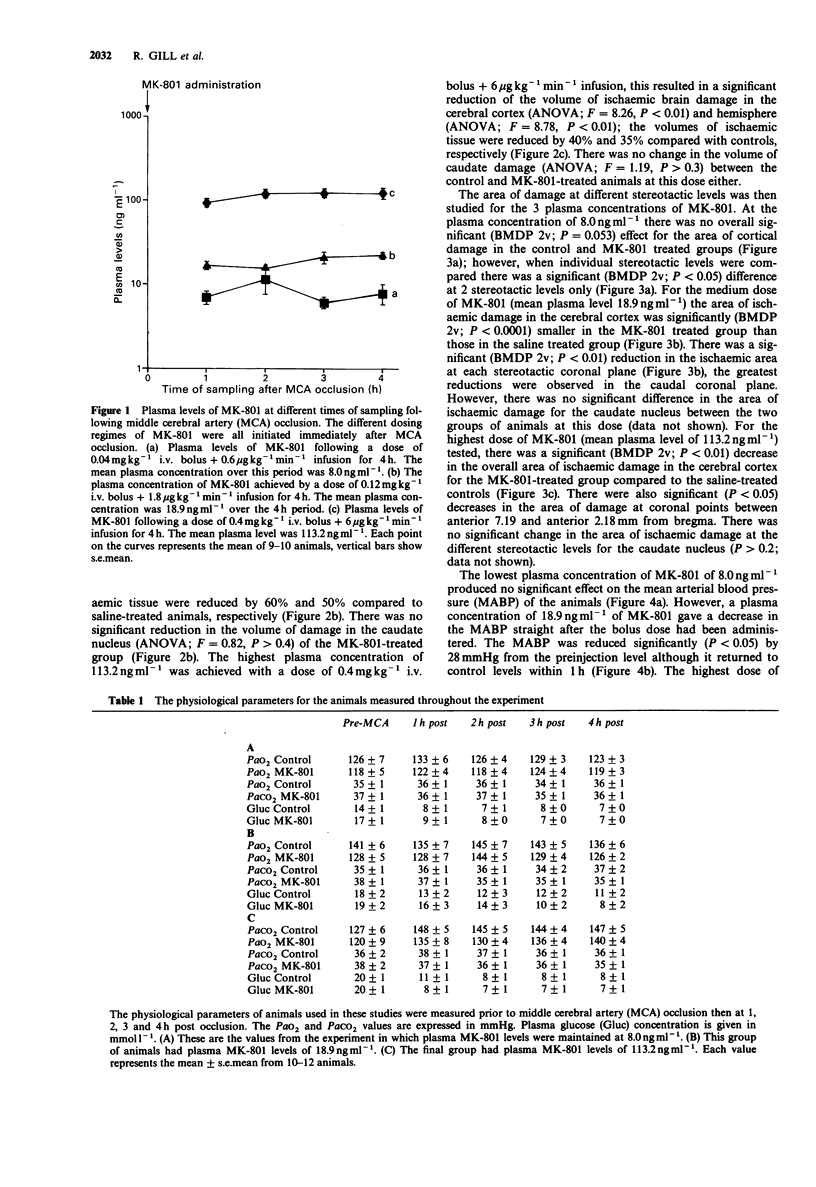
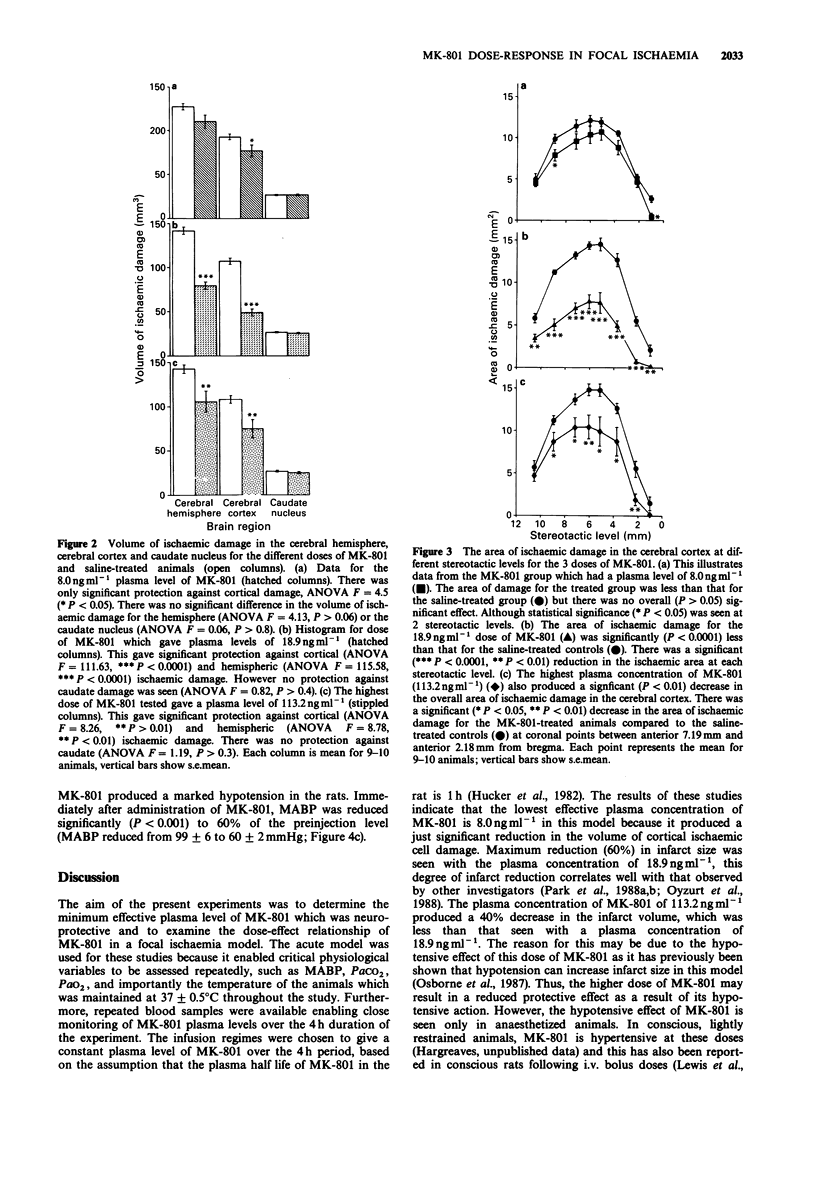
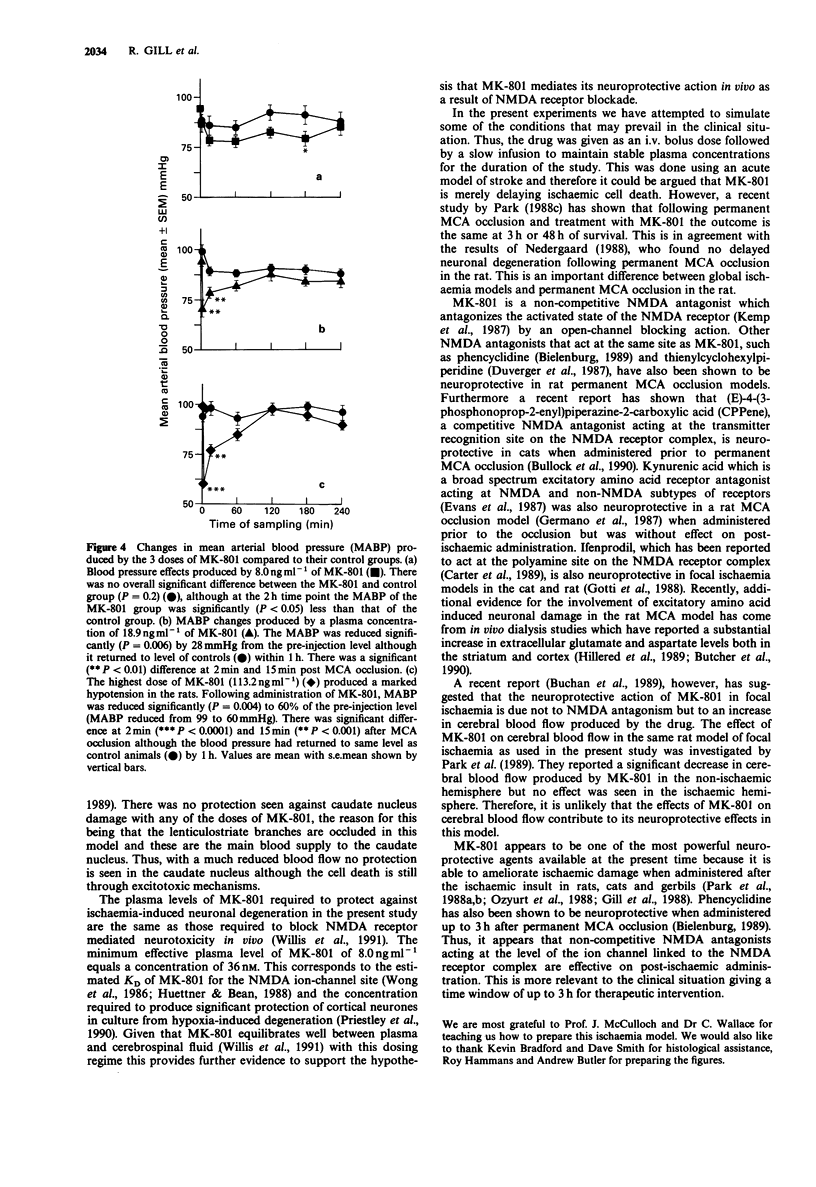
1. An acute model of focal ischaemia, which involves permanent occlusion of the middle cerebral artery of the rat with 4 h survival, was used to find the minimum effective plasma concentration of dizocilpine (MK-801) and to determine its dose-effect relationship. 2. MK-801 was administered at the time of occlusion and was given as an i.v. bolus followed by an infusion for 4 h to maintain a steady state plasma concentration of the drug throughout the study. MK-801 was given at 3 dose levels; 0.04 mg kg-1 i.v. bolus + 0.6 micrograms kg-1 min-1 infusion; 0.12 mg kg-1 i.v. bolus + 1.8 micrograms kg-1 min-1 infusion; 0.4 mg kg-1 i.v. bolus + 6 micrograms kg-1 min-1 infusion, which gave mean plasma levels over the 4 h of 8.0 ng ml-1, 18.9 ng ml-1 and 113.2 ng ml-1 respectively. 3. MK-801 at 8.0 ng ml-1 gave 10% reduction in the volume of ischaemic brain damage in the cerebral cortex which just reached significance. The middle dose of MK-801 (18.9 ng ml-1) gave a highly significant reduction in the volume of ischaemic brain damage in the cerebral cortex and hemisphere, volumes of ischaemic tissue being reduced by 60% and 50% compared to saline-treated animals, respectively. The highest plasma concentration of MK-801 (113.2 ng ml-1) resulted in a 35% reduction in the volume of hemispheric damage and a 40% reduction in the volume of cortical damage, which were significant.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andiné P., Jacobson I., Hagberg H. Calcium uptake evoked by electrical stimulation is enhanced postischemically and precedes delayed neuronal death in CA1 of rat hippocampus: involvement of N-methyl-D-aspartate receptors. J Cereb Blood Flow Metab. 1988 Dec;8(6):799–807. doi: 10.1038/jcbfm.1988.135. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Drejer J., Schousboe A., Diemer N. H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984 Nov;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Jørgensen M. B., Diemer N. H., Hansen A. J. Calcium accumulation by glutamate receptor activation is involved in hippocampal cell damage after ischemia. Acta Neurol Scand. 1988 Dec;78(6):529–536. doi: 10.1111/j.1600-0404.1988.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Jørgensen M. B., Sandberg M., Christensen T., Hagberg H., Diemer N. H. Ischemic damage in hippocampal CA1 is dependent on glutamate release and intact innervation from CA3. J Cereb Blood Flow Metab. 1989 Oct;9(5):629–639. doi: 10.1038/jcbfm.1989.90. [DOI] [PubMed] [Google Scholar]
- Boast C. A., Gerhardt S. C., Pastor G., Lehmann J., Etienne P. E., Liebman J. M. The N-methyl-D-aspartate antagonists CGS 19755 and CPP reduce ischemic brain damage in gerbils. Brain Res. 1988 Mar 1;442(2):345–348. doi: 10.1016/0006-8993(88)91522-3. [DOI] [PubMed] [Google Scholar]
- Bullock R., Graham D. I., Chen M. H., Lowe D., McCulloch J. Focal cerebral ischemia in the cat: pretreatment with a competitive NMDA receptor antagonist, D-CPP-ene. J Cereb Blood Flow Metab. 1990 Sep;10(5):668–674. doi: 10.1038/jcbfm.1990.120. [DOI] [PubMed] [Google Scholar]
- Butcher S. P., Bullock R., Graham D. I., McCulloch J. Correlation between amino acid release and neuropathologic outcome in rat brain following middle cerebral artery occlusion. Stroke. 1990 Dec;21(12):1727–1733. doi: 10.1161/01.str.21.12.1727. [DOI] [PubMed] [Google Scholar]
- Carter C., Rivy J. P., Scatton B. Ifenprodil and SL 82.0715 are antagonists at the polyamine site of the N-methyl-D-aspartate (NMDA) receptor. Eur J Pharmacol. 1989 May 30;164(3):611–612. doi: 10.1016/0014-2999(89)90275-6. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Evans S. J., Pook P. C., Sunter D. C. A comparison of excitatory amino acid antagonists acting at primary afferent C fibres and motoneurones of the isolated spinal cord of the rat. Br J Pharmacol. 1987 Jul;91(3):531–537. doi: 10.1111/j.1476-5381.1987.tb11246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano I. M., Pitts L. H., Meldrum B. S., Bartkowski H. M., Simon R. P. Kynurenate inhibition of cell excitation decreases stroke size and deficits. Ann Neurol. 1987 Dec;22(6):730–734. doi: 10.1002/ana.410220609. [DOI] [PubMed] [Google Scholar]
- Gill R., Foster A. C., Woodruff G. N. MK-801 is neuroprotective in gerbils when administered during the post-ischaemic period. Neuroscience. 1988 Jun;25(3):847–855. doi: 10.1016/0306-4522(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Gill R., Foster A. C., Woodruff G. N. Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. J Neurosci. 1987 Oct;7(10):3343–3349. doi: 10.1523/JNEUROSCI.07-10-03343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti B., Duverger D., Bertin J., Carter C., Dupont R., Frost J., Gaudilliere B., MacKenzie E. T., Rousseau J., Scatton B. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. I. Evidence for efficacy in models of focal cerebral ischemia. J Pharmacol Exp Ther. 1988 Dec;247(3):1211–1221. [PubMed] [Google Scholar]
- Hagberg H., Lehmann A., Sandberg M., Nyström B., Jacobson I., Hamberger A. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab. 1985 Sep;5(3):413–419. doi: 10.1038/jcbfm.1985.56. [DOI] [PubMed] [Google Scholar]
- Hichens M., Greber T. F., Vyas K. P. A radioimmunoassay for the anticonvulsant and neuroprotective agent, MK-801. J Immunoassay. 1990;11(4):477–502. doi: 10.1080/01971529008055046. [DOI] [PubMed] [Google Scholar]
- Hillered L., Hallström A., Segersvärd S., Persson L., Ungerstedt U. Dynamics of extracellular metabolites in the striatum after middle cerebral artery occlusion in the rat monitored by intracerebral microdialysis. J Cereb Blood Flow Metab. 1989 Oct;9(5):607–616. doi: 10.1038/jcbfm.1989.87. [DOI] [PubMed] [Google Scholar]
- Hucker H. B., Hutt J. E., White S. D., Arison B. H., Zacchei A. G. Disposition and metabolism of (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten-5,10-imine in rats, dogs, and monkeys. Drug Metab Dispos. 1983 Jan-Feb;11(1):54–58. [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. L., Auer R. N. Ketamine fails to protect against ischaemic neuronal necrosis in the rat. Br J Anaesth. 1988 Aug;61(2):206–210. doi: 10.1093/bja/61.2.206. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982 May 6;239(1):57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Lewis S. J., Barres C., Jacob H. J., Ohta H., Brody M. J. Cardiovascular effects of the N-methyl-D-aspartate receptor antagonist MK-801 in conscious rats. Hypertension. 1989 Jun;13(6 Pt 2):759–765. doi: 10.1161/01.hyp.13.6.759. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M. Mechanisms of brain damage in focal cerebral ischemia. Acta Neurol Scand. 1988 Feb;77(2):81–101. doi: 10.1111/j.1600-0404.1988.tb05878.x. [DOI] [PubMed] [Google Scholar]
- Osborne K. A., Shigeno T., Balarsky A. M., Ford I., McCulloch J., Teasdale G. M., Graham D. I. Quantitative assessment of early brain damage in a rat model of focal cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1987 Apr;50(4):402–410. doi: 10.1136/jnnp.50.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyurt E., Graham D. I., Woodruff G. N., McCulloch J. Protective effect of the glutamate antagonist, MK-801 in focal cerebral ischemia in the cat. J Cereb Blood Flow Metab. 1988 Feb;8(1):138–143. doi: 10.1038/jcbfm.1988.18. [DOI] [PubMed] [Google Scholar]
- Park C. K., Nehls D. G., Graham D. I., Teasdale G. M., McCulloch J. Focal cerebral ischaemia in the cat: treatment with the glutamate antagonist MK-801 after induction of ischaemia. J Cereb Blood Flow Metab. 1988 Oct;8(5):757–762. doi: 10.1038/jcbfm.1988.124. [DOI] [PubMed] [Google Scholar]
- Park C. K., Nehls D. G., Graham D. I., Teasdale G. M., McCulloch J. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann Neurol. 1988 Oct;24(4):543–551. doi: 10.1002/ana.410240411. [DOI] [PubMed] [Google Scholar]
- Park C. K., Nehls D. G., Teasdale G. M., McCulloch J. Effect of the NMDA antagonist MK-801 on local cerebral blood flow in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1989 Oct;9(5):617–622. doi: 10.1038/jcbfm.1989.88. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Feldmann E., Pulsinelli W. A., Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987 Aug;37(8):1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Priestley T., Horne A. L., McKernan R. M., Kemp J. A. The effect of NMDA receptor glycine site antagonists on hypoxia-induced neurodegeneration of rat cortical cell cultures. Brain Res. 1990 Oct 29;531(1-2):183–188. doi: 10.1016/0006-8993(90)90772-4. [DOI] [PubMed] [Google Scholar]
- Pulsinelli W. A., Brierley J. B. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979 May-Jun;10(3):267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Shigeno T., McCulloch J., Graham D. I., Mendelow A. D., Teasdale G. M. Pure cortical ischemia versus striatal ischemia. Circulatory, metabolic, and neuropathologic consequences. Surg Neurol. 1985 Jul;24(1):47–51. doi: 10.1016/0090-3019(85)90063-1. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1(2):155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Tamura A., Graham D. I., McCulloch J., Teasdale G. M. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1(1):53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Wieloch T., Lindvall O., Blomqvist P., Gage F. H. Evidence for amelioration of ischaemic neuronal damage in the hippocampal formation by lesions of the perforant path. Neurol Res. 1985 Mar;7(1):24–26. doi: 10.1080/01616412.1985.11739695. [DOI] [PubMed] [Google Scholar]
- Willis C. L., Brazell C., Foster A. C. Plasma and CSF levels of dizocilpine (MK-801) required for neuroprotection in the quinolinate-injected rat striatum. Eur J Pharmacol. 1991 Apr 24;196(3):285–290. doi: 10.1016/0014-2999(91)90441-r. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]


